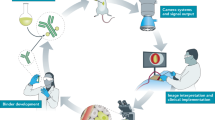
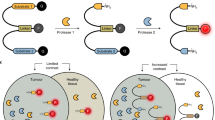
Abstract
The prognosis in advanced-stage ovarian cancer remains poor. Tumor-specific intraoperative fluorescence imaging may improve staging and debulking efforts in cytoreductive surgery and thereby improve prognosis. The overexpression of folate receptor-α (FR-α) in 90–95% of epithelial ovarian cancers prompted the investigation of intraoperative tumor-specific fluorescence imaging in ovarian cancer surgery using an FR-α–targeted fluorescent agent. In patients with ovarian cancer, intraoperative tumor-specific fluorescence imaging with an FR-α–targeted fluorescent agent showcased the potential applications in patients with ovarian cancer for improved intraoperative staging and more radical cytoreductive surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jemal, A. et al. Cancer statistics, 2008. CA Cancer J. Clin. 58, 71–96 (2008).
Gondos, A., Bray, F., Hakulinen, T. & Brenner, H. Trends in cancer survival in 11 European populations from 1990 to 2009: a model-based analysis. Ann. Oncol. 20, 564–573 (2009).
Aletti, G.D., Gallenberg, M.M., Cliby, W.A., Jatoi, A. & Hartmann, L.C. Current management strategies for ovarian cancer. Mayo Clin. Proc. 82, 751–770 (2007).
Winter, W.E. III et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 26, 83–89 (2008).
Ibeanu, O.A. & Bristow, R.E. Predicting the outcome of cytoreductive surgery for advanced ovarian cancer: a review. Int. J. Gynecol. Cancer 20 (suppl. 1), S1–S11 (2010).
Troyan, S.L. et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 16, 2943–2952 (2009).
Luker, G.D. & Luker, K.E. Optical imaging: current applications and future directions. J. Nucl. Med. 49, 1–4 (2008).
Tromberg, B.J. et al. Assessing the future of diffuse optical imaging technologies for breast cancer management. Med. Phys. 35, 2443–2451 (2008).
Kirsch, D.G. et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat. Med. 13, 992–997 (2007).
von Burstin, J. et al. Highly sensitive detection of early-stage pancreatic cancer by multimodal near-infrared molecular imaging in living mice. Int. J. Cancer 123, 2138–2147 (2008).
Sevick-Muraca, E.M. et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology 246, 734–741 (2008).
Tagaya, N. et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am. J. Surg. 195, 850–853 (2008).
Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401 (2006).
Kalli, K.R. et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 108, 619–626 (2008).
Markert, S. et al. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res. 28, 3567–3572 (2008).
Mathias, C.J. et al. Indium-111-DTPA-folate as a potential folate-receptor-targeted radiopharmaceutical. J. Nucl. Med. 39, 1579–1585 (1998).
Armstrong, D.K. et al. Exploratory phase II efficacy study of MORAb-003, a monoclonal antibody against folate receptor alpha, in platinum-sensitive ovarian cancer in first relapse. J. Clin. Oncol. 26, 5500 (2008).
Kennedy, M.D., Jallad, K.N., Thompson, D.H., Ben Amotz, D. & Low, P.S. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe. J. Biomed. Opt. 8, 636–641 (2003).
Leamon, C.P. et al. Synthesis and biological evaluation of EC20: a new folate-derived, 99mTc-based radiopharmaceutical. Bioconjug. Chem. 13, 1200–1210 (2002).
Themelis, G., Yoo, J.S., Soh, K.S., Schulz, R. & Ntziachristos, V. Real-time intraoperative fluorescence imaging system using light-absorption correction. J. Biomed. Opt. 14, 064012 (2009).
Sega, E.I. & Low, P.S. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 27, 655–664 (2008).
Crane, L.M.A. et al. The effect of chemotherapy on expression of folate receptor-α in ovarian cancer. Cell. Oncol. 34. Published online, doi: 10.1007/s13402-011-0052-6.
Vergote, I. et al. Timing of debulking surgery in advanced ovarian cancer. Int. J. Gynecol. Cancer 18 ( suppl. 1), 11–19 (2008).
Aletti, G.D. et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 107, 77–85 (2006).
Koppe, M.J., Boerman, O.C., Oyen, W.J. & Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann. Surg. 243, 212–222 (2006).
Low, P.S. & Antony, A.C. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv. Drug Deliv. Rev. 56, 1055–1058 (2004).
Parker, N. et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 338, 284–293 (2005).
Troy, T., Jekic-McMullen, D., Sambucetti, L. & Rice, B. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol. Imaging 3, 9–23 (2004).
Benedet, J.L., Bender, H., Jones, H. III, Ngan, H.Y. & Pecorelli, S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int. J. Gynaecol. Obstet. 70, 209–262 (2000).
Lu, Y. & Low, P.S. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol. Immunother. 51, 153–162 (2002).
Lu, Y. et al. Strategy to prevent drug-related hypersensitivity in folate-targeted hapten immunotherapy of cancer. AAPS J. 11, 628–638 (2009).
Themelis, G., Yoo, J.S. & Ntziachristos, V. Multispectral imaging using multiple-bandpass filters. Opt. Lett. 33, 1023–1025 (2008).
Acknowledgements
The authors wish to thank W. Sluiter for help with statistical analyses, I. Wesselman for training the operating room personnel in using the camera system and B. Molmans (Department of Hospital Pharmacy, University Medical Center Groningen) for pharmaceutical expertise and support. Monoclonal antibody mAb343 was kindly provided by W. Franklin (University of Colorado Health Science Center).
Author information
Authors and Affiliations
Contributions
G.M.v.D. supervised the project, designed and performed the study, interpreted data and wrote the manuscript. G.T. designed the camera system, performed the study, interpreted data with technical and computer analyses support and wrote the manuscript. L.M.A.C. designed and performed the study, interpreted data and wrote the manuscript. N.J.H. performed the study and interpreted data. R.G.P. performed the study and interpreted data. W.K. performed the study and interpreted data. A.S. designed the camera system, performed the study and interpreted data with technical and computer analysis support. J.S.d.J. performed the study and interpreted data. H.J.G.A. designed and performed the study, interpreted data and wrote the manuscript. A.G.J.v.d.Z. designed and performed the study, interpreted data and wrote the manuscript. J.B. designed and performed the study, interpreted data, performed the pathology analyses and wrote the manuscript. P.S.L. designed and performed the study, and interpreted data. V.N. supervised the project, designed and performed the study, designed and provided the camera system and interpreted data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Methods (PDF 220 kb)
Supplementary Video 1
Intraoperative application of the multispectral intraoperative fluorescence camera system (MOV 35948 kb)
Rights and permissions
About this article
Cite this article
van Dam, G., Themelis, G., Crane, L. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 17, 1315–1319 (2011). https://doi.org/10.1038/nm.2472
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2472
This article is cited by
-
Molecular image–guided surgery in gynaecological cancer: where do we stand?
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
High-precision detection and navigation surgery of colorectal cancer micrometastases
Journal of Nanobiotechnology (2023)
-
Prognostic value of peritoneal scar-like tissue in patients with peritoneal metastases of ovarian origin presenting for curative-intent cytoreductive surgery
World Journal of Surgical Oncology (2023)
-
Chemiluminescent probes in cancer biology
Nature Reviews Bioengineering (2023)
-
Fluorescence image-guided tumour surgery
Nature Reviews Bioengineering (2023)