Abstract
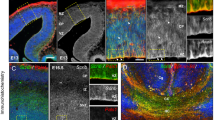
The ciliopathy Joubert syndrome is marked by cerebellar vermis hypoplasia, a phenotype for which the pathogenic mechanism is unclear1,2,3. To investigate Joubert syndrome pathogenesis, we have examined mice with mutated Ahi1, the first identified Joubert syndrome–associated gene4,5. These mice show cerebellar hypoplasia with a vermis-midline fusion defect early in development. This defect is concomitant with expansion of the roof plate and is also evident in a mouse mutant for another Joubert syndrome–associated gene, Cep2906,7. Furthermore, fetal magnetic resonance imaging (MRI) of human subjects with Joubert syndrome reveals a similar midline cleft, suggesting parallel pathogenic mechanisms. Previous evidence has suggested a role for Jouberin (Jbn), the protein encoded by Ahi1, in canonical Wnt signaling8. Consistent with this, we found decreased Wnt reporter activity at the site of hemisphere fusion in the developing cerebellum of Ahi1-mutant mice. This decrease was accompanied by reduced proliferation at the site of fusion. Finally, treatment with lithium, a Wnt pathway agonist9, partially rescued this phenotype. Our findings implicate a defect in Wnt signaling in the cerebellar midline phenotype seen in Joubert syndrome that can be overcome with Wnt stimulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Badano, J.L., Mitsuma, N., Beales, P.L. & Katsanis, N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 (2006).
Joubert, M., Eisenring, J.J., Robb, J.P. & Andermann, F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia and retardation. Neurology 19, 813–825 (1969).
Louie, C.M. & Gleeson, J.G. Genetic basis of Joubert syndrome and related disorders of cerebellar development. Hum. Mol. Genet. 14 Suppl. 2, R235–R242 (2005).
Dixon-Salazar, T. et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am. J. Hum. Genet. 75, 979–987 (2004).
Ferland, R.J. et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 36, 1008–1013 (2004).
Valente, E.M. et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 38, 623–625 (2006).
Sayer, J.A. et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 38, 674–681 (2006).
Lancaster, M.A. et al. Impaired Wnt–β-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 15, 1046–1054 (2009).
Hedgepeth, C.M. et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 185, 82–91 (1997).
Chizhikov, V.V. et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J. Neurosci. 27, 9780–9789 (2007).
Spassky, N. et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 317, 246–259 (2008).
Berbari, N.F., O'Connor, A.K., Haycraft, C.J. & Yoder, B.K. The primary cilium as a complex signaling center. Curr. Biol. 19, R526–R535 (2009).
Lancaster, M.A. & Gleeson, J.G. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 19, 220–229 (2009).
Louie, C.M. et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat. Genet. 42, 175–180 (2010).
Cooper, P.A., Benno, R.H., Hahn, M.E. & Hewitt, J.K. Genetic analysis of cerebellar foliation patterns in mice (Mus musculus). Behav. Genet. 21, 405–419 (1991).
Yachnis, A.T. & Rorke, L.B. Neuropathology of Joubert syndrome. J. Child Neurol. 14, 655–659, discussion 669–672 (1999).
Friede, R.L. & Boltshauser, E. Uncommon syndromes of cerebellar vermis aplasia. I: Joubert syndrome. Dev. Med. Child Neurol. 20, 758–763 (1978).
Corrales, J.D., Rocco, G.L., Blaess, S., Guo, Q. & Joyner, A.L. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131, 5581–5590 (2004).
Kenney, A.M., Cole, M.D. & Rowitch, D.H. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development 130, 15–28 (2003).
Chang, B. et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 15, 1847–1857 (2006).
Craige, B. et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927–940 (2010).
ten Donkelaar, H.J., Lammens, M., Wesseling, P., Thijssen, H.O. & Renier, W.O. Development and developmental disorders of the human cerebellum. J. Neurol. 250, 1025–1036 (2003).
Parisi, M.A. et al. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J. Med. Genet. 43, 334–339 (2006).
Valente, E.M. et al. AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann. Neurol. 59, 527–534 (2006).
Thomas, K.R., Musci, T.S., Neumann, P.E. & Capecchi, M.R. Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell 67, 969–976 (1991).
Schüller, U. & Rowitch, D.H. β-catenin function is required for cerebellar morphogenesis. Brain Res. 1140, 161–169 (2007).
Louvi, A., Alexandre, P., Metin, C., Wurst, W. & Wassef, M. The isthmic neuroepithelium is essential for cerebellar midline fusion. Development 130, 5319–5330 (2003).
Maretto, S. et al. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304 (2003).
Cheng, L.E., Zhang, J. & Reed, R.R. The transcription factor Zfp423/OAZ is required for cerebellar development and CNS midline patterning. Dev. Biol. 307, 43–52 (2007).
Millen, K.J., Wurst, W., Herrup, K. & Joyner, A.L. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development 120, 695–706 (1994).
Cohen, E.D. et al. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J. Clin. Invest. 119, 2538–2549 (2009).
Marino, S. Medulloblastoma: developmental mechanisms out of control. Trends Mol. Med. 11, 17–22 (2005).
Hsiao, Y.C. et al. Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum. Mol. Genet. 18, 3926–3941 (2009).
Kim, J., Krishnaswami, S.R. & Gleeson, J.G. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 17, 3796–3805 (2008).
Grimmer, M.R. & Weiss, W.A. BMPs oppose Math1 in cerebellar development and in medulloblastoma. Genes Dev. 22, 693–699 (2008).
Yaguchi, Y. et al. Fibroblast growth factor (FGF) gene expression in the developing cerebellum suggests multiple roles for FGF signaling during cerebellar morphogenesis and development. Dev. Dyn. 238, 2058–2072 (2009).
Koizumi, H., Tanaka, T. & Gleeson, J.G. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron 49, 55–66 (2006).
Saleem, S.N. & Zaki, M.S. Role of MR imaging in prenatal diagnosis of pregnancies at risk for Joubert syndrome and related cerebellar disorders. AJNR Am. J. Neuroradiol. 31, 424–429 (2010).
Hatten, M.E. Neuronal regulation of astroglial morphology and proliferation in vitro. J. Cell Biol. 100, 384–396 (1985).
Lee, J.K. et al. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J. Neurosci. 29, 8649–8654 (2009).
Acknowledgements
We are grateful to members of the Gleeson lab for technical expertise and feedback and B. Hamilton, M. Hatten and A. Joyner for helpful discussions on cerebellar development. We also thank the study subjects and the UCSD Neuroscience Microscopy Core. We thank J.K. Lee and B. Zheng for help with the biotinylated dextran amine tracing assay. We are grateful to S. Piccolo (University of Padua) for the BATgal mice. We thank R.T. Moon (University of Washington) for the Super Topflash construct and P. Mellon (UCSD) for the β-galactosidase expression construct, as well as K. Willert (UCSD) for stably transfected L cells and the β-catenin expression plasmid. M.A.L. received support from the Bear Necessities Pediatric Cancer Foundation and US National Institutes of Health–National Institute of General Medical Sciences–funded UCSD Genetics Training Program (T32 GM08666). This work was supported by the US National Institutes of Health (grants P30NS047101, R01NS052455 and R01NS048453) and the Burroughs Wellcome Fund in Translational Research (J.G.G.). J.G.G. is an investigator with Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.G.G. and M.A.L. conceived of and designed the experimental approach, interpreted data and wrote the manuscript. M.A.L. and D.J.G. carried out in vivo characterization experiments. M.A.L. performed in vitro Wnt assays. J.K. and Y.W. designed and generated Cep290-mutant mice. S.N.S. and M.S.Z. performed human diagnoses and provided MRIs. J.L.S. generated constructs and materials for in vitro assays. C.M.L. and B.E.T. contributed to in vivo characterizations. J.G.G. directed and supervised the project.
Corresponding author
Ethics declarations
Competing interests
J.G.G. consults for Novartis in the area of ciliopathy therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Discussion (PDF 776 kb)
Rights and permissions
About this article
Cite this article
Lancaster, M., Gopal, D., Kim, J. et al. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat Med 17, 726–731 (2011). https://doi.org/10.1038/nm.2380
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2380
This article is cited by
-
Abelson Helper Integration Site 1 haplotypes and peripheral blood expression associates with lithium response and immunomodulation in bipolar patients
Psychopharmacology (2024)
-
Developmental and foliation changes due to dysregulation of adenosine kinase in the cerebellum
Scientific Reports (2023)
-
Cerebellum Lecture: the Cerebellar Nuclei—Core of the Cerebellum
The Cerebellum (2023)
-
Diagnostic Approach to Cerebellar Hypoplasia
The Cerebellum (2021)
-
Identification of a novel truncating variant in AHI1 gene and a brief review on mutations spectrum
Molecular Biology Reports (2021)