Abstract
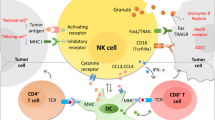
The natural killer (NK) cell receptor NKp30 is involved in the recognition of tumor and dendritic cells (DCs). Here we describe the influence of three NKp30 splice variants on the prognosis of gastrointestinal sarcoma (GIST), a malignancy that expresses NKp30 ligands and that is treated with NK-stimulatory KIT tyrosine kinase inhibitors. Healthy individuals and those with GIST show distinct patterns of transcription of functionally different NKp30 isoforms. In a retrospective analysis of 80 individuals with GIST, predominant expression of the immunosuppressive NKp30c isoform (over the immunostimulatory NKp30a and NKp30b isoforms) was associated with reduced survival of subjects, decreased NKp30-dependent tumor necrosis factor-α (TNF-α) and CD107a release, and defective interferon-γ (IFN-γ) and interleukin-12 (IL-12) secretion in the NK-DC cross-talk that could be restored by blocking of IL-10. Preferential NKp30c expression resulted partly from a single-nucleotide polymorphism at position 3790 in the 3′ untranslated region of the gene encoding NKp30. The genetically determined NKp30 status predicts the clinical outcomes of individuals with GIST independently from KIT mutation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hirota, S. et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998).
Heinrich, M.C. et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 21, 4342–4349 (2003).
MetaGIST. Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J. Clin. Oncol. 28, 1247–1253 (2010).
Borg, C. et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell–dependent antitumor effects. J. Clin. Invest. 114, 379–388 (2004).
Ménard, C. et al. Natural killer cell IFN-γ levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 69, 3563–3569 (2009).
Taieb, J. et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 12, 214–219 (2006).
Yutkin, V., Pode, D., Pikarsky, E. & Mandelboim, O. The expression level of ligands for natural killer cell receptors predicts response to bacillus Calmette-Guerin therapy: a pilot study. J. Urol. 178, 2660–2664 (2007).
De Maria, A. et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur. J. Immunol. 37, 445–455 (2007).
Vankayalapati, R. et al. Role of NK cell–activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. 175, 4611–4617 (2005).
Chisholm, S.E., Howard, K., Gomez, M.V. & Reyburn, H.T. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J. Infect. Dis. 195, 1160–1168 (2007).
Rutjens, E. et al. Differential NKp30 inducibility in chimpanzee NK cells and conserved NK cell phenotype and function in long-term HIV-1–infected animals. J. Immunol. 178, 1702–1712 (2007).
Hermann, E. et al. Human congenital infection with Trypanosoma cruzi induces phenotypic and functional modifications of cord blood NK cells. Pediatr. Res. 60, 38–43 (2006).
Fuller, C.L. et al. NKp30-dependent cytolysis of filovirus-infected human dendritic cells. Cell. Microbiol. 9, 962–976 (2007).
Mavoungou, E., Held, J., Mewono, L. & Kremsner, P.G. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum–parasitized erythrocytes by natural killer cells. J. Infect. Dis. 195, 1521–1531 (2007).
Mavilio, D. et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J. Exp. Med. 203, 2339–2350 (2006).
Poggi, A. et al. NKG2D and natural cytotoxicity receptors are involved in natural killer cell interaction with self-antigen presenting cells and stromal cells. Ann. NY Acad. Sci. 1109, 47–57 (2007).
Fauriat, C. et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 109, 323–330 (2007).
Ferlazzo, G. et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195, 343–351 (2002).
Pende, D. et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190, 1505–1516 (1999).
Vitale, M. et al. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood 106, 566–571 (2005).
Gruen, J.R. & Weissman, S.M. Human MHC class III and IV genes and disease associations. Front. Biosci. 6, D960–D972 (2001).
Nalabolu, S.R., Shukla, H., Nallur, G., Parimoo, S. & Weissman, S.M. Genes in a 220-kb region spanning the TNF cluster in human MHC. Genomics 31, 215–222 (1996).
Neville, M.J. & Campbell, R.D. A new member of the Ig superfamily and a V-ATPase G subunit are among the predicted products of novel genes close to the TNF locus in the human MHC. J. Immunol. 162, 4745–4754 (1999).
Sivakamasundari, R., Raghunathan, A., Zhang, C.Y., Chowdhury, R.R. & Weissman, S.M. Expression and cellular localization of the protein encoded by the 1C7 gene: a recently described component of the MHC. Immunogenetics 51, 723–732 (2000).
Hollyoake, M., Campbell, R.D. & Aguado, B. NKp30 (NCR3) is a pseudogene in 12 inbred and wild mouse strains, but an expressed gene in Mus caroli. Mol. Biol. Evol. 22, 1661–1672 (2005).
Xie, T. et al. Analysis of the gene-dense major histocompatibility complex class III region and its comparison to mouse. Genome Res. 13, 2621–2636 (2003).
Miettinen, M., Majidi, M. & Lasota, J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur. J. Cancer 38 Suppl 5, S39–S51 (2002).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Robertson, M.J. et al. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 24, 406–415 (1996).
Brandt, C.S. et al. The B7 family member B7–H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 206, 1495–1503 (2009).
Pandey, R., DeStephan, C.M., Madge, L.A., May, M.J. & Orange, J.S. NKp30 ligation induces rapid activation of the canonical NF-κB pathway in NK cells. J. Immunol. 179, 7385–7396 (2007).
Grant, L.R. et al. Stat4-dependent, T-bet–independent regulation of IL-10 in NK cells. Genes Immun. 9, 316–327 (2008).
Perona-Wright, G. et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe 6, 503–512 (2009).
Vivier, E. & Ugolini, S. Regulatory natural killer cells: new players in the IL-10 anti-inflammatory response. Cell Host Microbe 6, 493–495 (2009).
Emile, J.F. et al. Length analysis of polymerase chain reaction products: a sensitive and reliable technique for the detection of mutations in KIT exon 11 in gastrointestinal stromal tumors. Diagn. Mol. Pathol. 11, 107–112 (2002).
Sheets, M.D., Ogg, S.C. & Wickens, M.P. Point mutations in AAUAAA and the poly (A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acids Res. 18, 5799–5805 (1990).
Pogge von Strandmann, E. et al. Human leukocyte antigen-B–associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27, 965–974 (2007).
Deniz, G. et al. Regulatory NK cells suppress antigen-specific T cell responses. J. Immunol. 180, 850–857 (2008).
Teng, M.W. et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc. Natl. Acad. Sci. USA 107, 8328–8333 (2010).
Vitale, M., Sivori, S., Pende, D., Moretta, L. & Moretta, A. Coexpression of two functionally independent p58 inhibitory receptors in human natural killer cell clones results in the inability to kill all normal allogeneic target cells. Proc. Natl. Acad. Sci. USA 92, 3536–3540 (1995).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) Method. Methods 25, 402–408 (2001).
Eisen, M.B., Spellman, P.T., Brown, P.O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
Delahaye, N.F., Barbier, M., Fumoux, F. & Rihet, P. Association analyses of NCR3 polymorphisms with P. falciparum mild malaria. Microbes Infect. 9, 160–166 (2007).
Acknowledgements
We thank P. Anderson for providing the CD3ζ-specific antibody (2H2 clone), M. Baratin for helpful discussions and the affected subjects as well as the healthy volunteers for their kind participation. U848 and U1015 are supported by Ligue contre le Cancer (équipes labellisées), Fondation pour la Recherche Médicale, European Union (Apo-Sys, ArtForce, ChemoRes, INFLACARE), INCa ('NKp30' in 2006; 'NCR3 isoforms' in 2008), Agence Nationale de la Recherche and Fondation de France (2009–2011). N.F.D. was supported by Institut National du Cancer and Fondation Gustave Roussy. S. Rusakiewicz was supported by a studentship from the Fondation pour la Recherche Médicale. V.B. was supported by the Ligue contre le Cancer, Agence Nationale de la Recherche, Association pour la Recherche contre le Cancer, Belgian Interuniversity Attraction Pole, Cancéropole Ile-de-France and Université Paris Descartes. J.C.G. was supported by the Fundayacucho-CNOUS joint program. Some materials and data used in this study were provided by the conticagist (https://www.conticagist.org/).
Author information
Authors and Affiliations
Contributions
N.F.D., S. Ruskiewicz, I.M., C.M., S. Roux, N.C., M. Sarabi, C.F., M. Semeraro, V.P.-C. and K.C. performed the experiments. L.L. and P.P. did qRT-PCRs. V.M.-C. and D.V.-C. contributed to clinical aspects of the study. V.B. and H.A. performed the electrophoretic mobility shift assays. S.K.-R. and M.P. provided the p38 MAP kinase inhibitors and scientific advice. I.C. contributed to the NKp46 immunohistochemistry study. L.P. and B.R. performed single-cell RT-PCRs. C.B. and A.M. provided the NK cell clones and the NKp30 and NKp44 antibodies. J.C.G., J.A.N. and J.T. contributed to exploration of the MAP kinase pathway. F.C. contributed to the statistical analysis. N. Ibrahim, P.T. and P.O. provided the paraffin-embedded GIST specimens and monitored histopathology data. S.B., J.-M.C., J.-Y.B., N. Isambert and A.L. provided samples from affected individuals and clinical data. J.-F.E. identified the KIT mutations in the affected-subject cohort. P.R. contributed to genotyping of the NCR3 mutations. E.V. provided the B7H6 antibody and scientific advice. N.F.D., S. Rusakiewicz and I.M. prepared the figures and drafted the manuscript. G.K. and L.Z. designed the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–3 and Supplementary Methods (PDF 696 kb)
Rights and permissions
About this article
Cite this article
Delahaye, N., Rusakiewicz, S., Martins, I. et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 17, 700–707 (2011). https://doi.org/10.1038/nm.2366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2366
This article is cited by
-
Dynamic changes of phenotype and function of natural killer cells in peripheral blood before and after thermal ablation of hepatitis B associated hepatocellular carcinoma and their correlation with tumor recurrence
BMC Cancer (2023)
-
Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers
Molecular Cancer (2023)
-
Peripheral blood-based cell signature indicates response to interstitial brachytherapy in primary liver cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Exploring Natural Killer Cell Testing in Embryo Implantation and Reproductive Failure: An Overview of Techniques and Controversies
Reproductive Sciences (2023)
-
NK cells and solid tumors: therapeutic potential and persisting obstacles
Molecular Cancer (2022)