Abstract
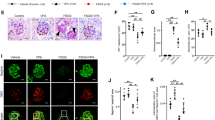
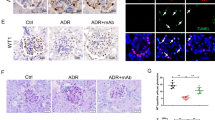
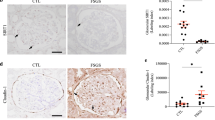
The main manifestations of nephrotic syndrome include proteinuria, hypoalbuminemia, edema, hyperlipidemia and lipiduria. Common causes of nephrotic syndrome are diabetic nephropathy, minimal change disease (MCD), focal and segmental glomerulosclerosis (FSGS) and membranous nephropathy. Among the primary glomerular diseases, MCD is usually sensitive to glucocorticoid treatment, whereas the other diseases show variable responses1. Despite the identification of key structural proteins in the glomerular capillary loop which may contribute to defects in ultrafiltration, many of the disease mechanisms of nephrotic syndrome remain unresolved. In this study, we show that the glomerular expression of angiopoietin-like-4 (Angptl4), a secreted glycoprotein, is glucocorticoid sensitive and is highly upregulated in the serum and in podocytes in experimental models of MCD and in the human disease. Podocyte-specific transgenic overexpression of Angptl4 (NPHS2-Angptl4) in rats induced nephrotic-range, and selective, proteinuria (over 500-fold increase in albuminuria), loss of glomerular basement membrane (GBM) charge and foot process effacement, whereas transgenic expression specifically in the adipose tissue (aP2-Angptl4) resulted in increased circulating Angptl4, but no proteinuria. Angptl4−/− mice that were injected with lipopolysaccharide (LPS) or nephritogenic antisera developed markedly less proteinuria than did control mice. Angptl4 secreted from podocytes in some forms of nephrotic syndrome lacks normal sialylation. When we fed the sialic acid precursor N-acetyl-D-mannosamine (ManNAc) to NPHS2-Angptl4 transgenic rats it increased the sialylation of Angptl4 and decreased albuminuria by more than 40%. These results suggest that podocyte-secreted Angptl4 has a key role in nephrotic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Nachman, P.H., Jennette, J.C. & Falk, R. Primary glomerular disease. in The Kidney 8th edn. (ed. Brenner, B.M.) 987–1066 (Elsevier, Philadelphia, 2008).
Chugh, S. et al. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int. 59, 601–613 (2001).
Liu, G., Clement, L., Kanwar, Y.S., Avila-Casado, C. & Chugh, S.S. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J. Biol. Chem. 281, 39681–39692 (2006).
Yoshida, K., Shimizugawa, T., Ono, M. & Furukawa, H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43, 1770–1772 (2002).
Padua, D. et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008).
Kersten, S. et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator–activated receptor target gene. J. Biol. Chem. 275, 28488–28493 (2000).
Yoon, J.C. et al. Peroxisome proliferator–activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20, 5343–5349 (2000).
Kim, I. et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem. J. 346, 603–610 (2000).
Ge, H. et al. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J. Biol. Chem. 279, 2038–2045 (2004).
Ge, H., Yang, G., Yu, X., Pourbahrami, T. & Li, C. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. J. Lipid Res. 45, 2071–2079 (2004).
Romeo, S. et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39, 513–516 (2007).
Romeo, S. et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119, 70–79 (2009).
Mandard, S. et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281, 934–944 (2006).
Avila-Casado, C. et al. Proteinuria in rats induced by serum from patients with collapsing glomerulopathy. Kidney Int. 66, 133–143 (2004).
Clement, L. et al. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 72, 337–347 (2007).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Davis, B. et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J. Am. Soc. Nephrol. 18, 2320–2329 (2007).
Cazes, A. et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ. Res. 99, 1207–1215 (2006).
Malicdan, M.C., Noguchi, S., Hayashi, Y.K., Nonaka, I. & Nishino, I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat. Med. 15, 690–695 (2009).
Galeano, B. et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J. Clin. Invest. 117, 1585–1594 (2007).
Koliwad, S.K. et al. Angiopoietin-like 4 (ANGPTL4/FIAF) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J. Biol. Chem. 284, 25593–25601 (2009).
Graves, R.A., Tontonoz, P., Platt, K.A., Ross, S.R. & Spiegelman, B.M. Identification of a fat cell enhancer: analysis of requirements for adipose tissue–specific gene expression. J. Cell. Biochem. 49, 219–224 (1992).
Acknowledgements
We thank B. Spiegelman (Dana-Farber Cancer Institute) for the mouse aP2 promoter construct; L. Holzman (University of Michigan) for the human NPHS2 promoter construct; P. Mundel (University of Miami) for mouse GECs; T. van Kuppevelt (Radboud University Nijmegen Medical Center) for the heparan sulfate proteoglycan–specific antibody p1113; D. Salant (Boston University) for γ2-NTS; and A. Köster (Eli Lilly) for Angptl4−/− mice. We also thank G. Bai for the generation of probe for in situ hybridization; S. Shastry for some ELISA assays for rat and mouse albumin; S. Rahmanuddin for selected immunogold studies and polyethyleneimine studies; F. Mattijssen for mouse LPS study; J. Novak for advice on choice of lectins; and Y.S. Kanwar and M. Venkatachalam for useful discussions on heparan sulfate proteoglycans. Supported by the US National Institutes of Health (R56DK077073, R01DK077073 to S.S.C.), Norman S. Coplon Satellite Research Grant to S.S.C. and CONACYT 111 grant to C.A.-C. We thank the University of Alabama at Birmingham–University of California–San Diego George O'Brien Center Core C for measuring mouse urine creatinine by mass spectrometry. The use of sialic acid precursors, including ManNAc, to treat proteinuria and nephrotic syndrome is covered by US Provisional Application 61/351,865 filed by S.S.C.
Author information
Authors and Affiliations
Contributions
L.C.C. was lead postdoctoral fellow, conducted most of the animal studies, generated stable cell lines and purified recombinant protein, maintained transgenic rats and conducted all studies on transgenic rats, confocal imaging and in situ hybridization, most gene expression studies and selected two-dimensional gel studies. C.A.-C. interpreted and analyzed light microscopy, electron microscopy and immunogold electron microscopy sections for the study and conducted studies on induction of collapsing glomerulopathy in rats. C.M. conducted most of the two-dimensional gel electrophoresis and proteomic work, and most of the albumin ELISA assays. E.S. acted as electron microscopist and morphometrics expert, prepared tissue for electron microscopy, conducted and imaged conventional and most immunogold electron microscopy studies, and conducted alcian blue charge studies. W.W.B. provided human sera and assisted in study design and preparation of the manuscript. S.K. conducted studies on Angptl4 transgenic mice, provided tissue for histological and gene expression analysis, conducted Angptl4−/− mouse studies with NTS and made substantial contributions to the preparation of the manuscript. S.S.C. acted as senior investigator, planned and supervised the study, generated constructs for transgenic rats, conducted molecular biology and gene expression studies, conducted early animal studies, and wrote and revised the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, Supplementary Tables 1 and 2 and Supplementary Methods (PDF 1867 kb)
Rights and permissions
About this article
Cite this article
Clement, L., Avila-Casado, C., Macé, C. et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17, 117–122 (2011). https://doi.org/10.1038/nm.2261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2261
This article is cited by
-
Higher circulating levels of non-esterified fatty acids are associated with faster kidney function decline in post-menopausal women with type 2 diabetes: a pilot prospective study
Acta Diabetologica (2023)
-
ANGPTL4 negatively regulates the progression of osteosarcoma by remodeling branched-chain amino acid metabolism
Cell Death Discovery (2022)
-
How immunosuppressive drugs may directly target podocytes in glomerular diseases
Pediatric Nephrology (2022)
-
Serum and urine ANGPTL8 expression levels are associated with hyperlipidemia and proteinuria in primary nephrotic syndrome
BMC Nephrology (2021)
-
Circulating ANGPTL8 levels and risk of kidney function decline: Results from the 4C Study
Cardiovascular Diabetology (2021)