Abstract
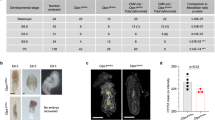
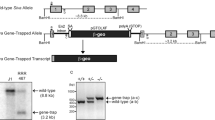
Apoptosis is often accompanied by the degradation of chromosomal DNA. Caspase-activated DNase (CAD) is an endonuclease that is activated in dying cells, whereas DNase II is present in the lysosomes of macrophages. Here, we show that CAD−/− thymocytes did not undergo apoptotic DNA degradation. But, when apoptotic cells were phagocytosed by macrophages, their DNA was degraded by DNase II. The thymus of DNase II−/−CAD−/− embryos contained many foci carrying undigested DNA and the cellularity was severely reduced due to a block in T cell development. The interferon-β gene was strongly up-regulated in the thymus of DNase II−/−CAD−/− embryos, suggesting that when the DNA of apoptotic cells is left undigested, it can activate innate immunity leading to defects in thymic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kerr, J.F., Wyllie, A.H. & Currie, A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Jacobson, M.D., Weil, M. & Raff, M.C. Programmed cell death in animal development. Cell 88, 347–354 (1997).
Vaux, D.L. & Korsmeyer, S.J. Cell death in development. Cell 96, 245–254 (1999).
Raff, M. Cell suicide for beginners. Nature 396, 119–122 (1998).
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
Ashkenazi, A. & Dixit, V.M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Green, D.R. & Reed, J.C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Thornberry, N.A. & Lazebnik, Y. Caspases: enemies within. Science 281, 1312–1316 (1998).
Earnshaw, W.C., Martins, L.M. & Kaufmann, S.H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68, 383–424 (1999).
Los, M., Wesselborg, S. & Schulze-Osthoff, K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 10, 629–639 (1999).
Savill, J. & Fadok, V. Corpse clearance defines the meaning of cell death. Nature 407, 784–788 (2000).
Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284, 555–556 (1980).
Nagata, S., Nagase, H., Kawane, K., Mukae, N. & Fukuyama, H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. (in the press).
Enari, M. et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391, 43–50 (1998).
Liu, X., Zou, H., Slaughter, C. & Wang, X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89, 175–184 (1997).
Sakahira, H., Enari, M. & Nagata, S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391, 96–99 (1998).
McCarty, J.S., Toh, S.Y. & Li, P. Multiple domains of DFF45 bind synergistically to DFF40: roles of caspase cleavage and sequestration of activator domain of DFF40. Biochem. Biophys. Res. Commun. 264, 181–185 (1999).
Sakahira, H. & Nagata, S. Co-translational folding of caspase-activated DNase with Hsp70, Hsp40 and inhibitor of caspase-activated DNase. J. Biol. Chem. 277, 3364–3370 (2002).
Zhang, J. et al. Resistance to DNA fragmentation and chromatin condensation in mice lacking the DNA fragmentation factor 45. Proc. Natl. Acad. Sci. USA 95, 12480–12485 (1998).
McIlroy, D. et al. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 14, 549–558 (2000).
Li, L.Y., Luo, X. & Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412, 95–99 (2001).
Parrish, J. et al. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature 412, 90–94 (2001).
van Loo, G. et al. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 8, 1136–1142 (2001).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Bernardi, G. in The Enzymes (ed. Boyer, P.D.) 271–287 (Academic Press, New York and London, 1971).
Kawane, K. et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science 292, 1546–1549 (2001).
Krieser, R.J. et al. Deoxyribonuclease IIa is required during the phagocytic phase of apoptosis and its loss causes lethality. Cell Death Differ. 9, 956–962 (2002).
Kawane, K. et al. Structure and promoter analysis of murine CAD and ICAD genes. Cell Death Differ. 6, 745–752 (1999).
Oberhammer, F. et al. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 12, 3679–3684 (1993).
Godfrey, D.I., Kennedy, J., Suda, T. & Zlotnik, A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 150, 4244–4252 (1993).
Yaegashi, Y., Nielsen, P., Sing, A., Galanos, C. & Freudenberg, M.A. Interferon β, a cofactor in the interferon γ production induced by gram-negative bacteria in mice. J. Exp. Med. 181, 953–960 (1995).
Su, D.M., Wang, J., Lin, Q., Cooper, M.D. & Watanabe, T. Interferons α/β inhibit IL-7-induced proliferation of CD4− CD8− CD3− CD44+ CD25+ thymocytes, but do not inhibit that of CD4− CD8− CD3− CD44− CD25− thymocytes. Immunology 90, 543–549 (1997).
Lin, Q., Dong, C. & Cooper, M.D. Impairment of T and B cell development by treatment with a type I interferon. J. Exp. Med. 187, 79–87 (1998).
Montgomery, R.A. & Dallman, M.J. Semi-quantitative polymerase chain reaction analysis of cytokine and cytokine receptor gene expression during thymic ontogeny. Cytokine 9, 717–726 (1997).
Arends, M.J., Morris, R.G. & Wyllie, A.H. The role of the endonuclease. Am. J. Pathol. 136, 593–608 (1993).
Gavrieli, Y., Sherman, Y. & Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493–501 (1992).
Staley, K., Blaschke, A. & Chun, J. Apoptotic DNA fragmentation is detected by a semi-quantitative ligation-mediated PCR of blunt DNA ends. Cell Death Differ. 4, 66–75 (1997).
Peitsch, M.C. et al. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 12, 371–377 (1993).
Susin, S.A. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446 (1999).
Wu, Y.C., Stanfield, G.M. & Horvitz, H.R. NUC-1, a Caenorhabditis elegans DNase II homolog, functions in an intermediate step of DNA degradation during apoptosis. Genes Dev. 14, 536–548 (2000).
Mukae, N., Yokoyama, H., Yokokura, T., Sakoyama, Y. & Nagata, S. Activation of the innate immunity in Drosophila by endogenous chromosomal DNA that escaped apoptotic degradation. Genes Dev. 16, 2662–2671 (2002).
Durrieu, F. et al. Caspase activation is an early event in anthracycline-induced apoptosis and allows detection of apoptotic cells before they are ingested by phagocytes. Exp. Cell Res. 240, 165–175 (1998).
Binder, D., Fehr, J., Hengartner, H. & Zinkernagel, R.M. Virus-induced transient bone marrow aplasia: major role of interferon-α/β during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J. Exp. Med. 185, 517–530 (1997).
Doly, J., Civas, A., Navarro, S. & Uze, G. Type I interferons: expression and signalization. Cell Mol. Life Sci. 54, 1109–1121 (1998).
Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20, 709–760 (2002).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Bird, A.P. CpG-rich islands and the function of DNA methylation. Nature 321, 209–213 (1986).
Leadbetter, E.A. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Kondoh, G. et al. Easy assessment of ES cell clone potency for chimeric development and germ-line competency by an optimized aggregation method. J. Biochem. Biophys. Methods 39, 137–142 (1999).
Platt, N., Suzuki, H., Kurihara, Y., Kodama, T. & Gordon, S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. USA 93, 12456–12460 (1996).
Takeshita, S., Kaji, K. & Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488 (2000).
Laird, P.W. et al. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 19, 4293 (1991).
Sambrook, J. & Russell, D.W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 2001).
Acknowledgements
We thank M. Adachi for help in the initial stage of this study; K. Miwa for PCR; A. Kudo for CMG-12 cells; K. Ishihara for the advice on the fetal thymus organ culture; and S. Aoyama and M. Harayama for secretarial assistance. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture in Japan. K.K. is supported by a research fellowship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kawane, K., Fukuyama, H., Yoshida, H. et al. Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat Immunol 4, 138–144 (2003). https://doi.org/10.1038/ni881
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni881
This article is cited by
-
Neutrophil extracellular traps have auto-catabolic activity and produce mononucleosome-associated circulating DNA
Genome Medicine (2022)
-
Spontaneous activity of the mitochondrial apoptosis pathway drives chromosomal defects, the appearance of micronuclei and cancer metastasis through the Caspase-Activated DNAse
Cell Death & Disease (2022)
-
The Role of Extracellular DNA and Histones in Ischaemia-Reperfusion Injury of the Myocardium
Cardiovascular Drugs and Therapy (2020)
-
Delicate regulation of the cGAS–MITA-mediated innate immune response
Cellular & Molecular Immunology (2018)
-
Apoptosis regulation by subcellular relocation of caspases
Scientific Reports (2018)