Abstract
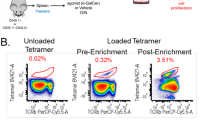
CD1d-reactive natural killer T (NKT) cells with an invariant Vα14 rearrangement (Vα14i) are a distinct subset of T lymphocytes that likely have important immune-regulatory functions. Little is known regarding the factors responsible for their peripheral survival. Using α-galactosylceramide–containing CD1d tetramers to detect Vα14i NKT cells, we show here that the expansion of Vα14i NKT cells in lymphopenic mice was not dependent on CD1d expression and was unaffected by the presence of host NKT cells. Additionally, we found that IL-15 was important in the expansion and/or survival of Vα14i NKT cells, with IL-7 playing a lesser role. These results demonstrate that the homeostatic requirements for CD1d-restricted NKT cells, which are CD4+ or CD4−CD8−, resemble those of CD8+ memory T cells. We propose that this expansion and/or survival in the periphery of Vα14i NKT cells is affected by competition for IL-15, and that IL-15–requiring cells—such as NK cells and CD8+ memory cells—may define the Vα14i NKT cell niche.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Tough, D.F. & Sprent, J. Life span of naive and memory T cells. Stem Cells 13, 242–249 (1995).
Jameson, S.C. Maintaining the norm: T-cell homeostasis. Nature Rev. Immunol. 2, 547–556 (2002).
Goldrath, A.W. & Bevan, M.J. Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 (1999).
Marrack, P. et al. Homeostasis of αβ TCR+ T cells. Nature Immunol. 1, 107–111 (2000).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 (1999).
Swain, S.L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999).
Prlic, M., Lefrancois, L. & Jameson, S.C. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J. Exp. Med. 195, 49–52 (2002).
Ma, A., Boone, D.L. & Lodolce, J.P. The pleiotropic functions of interleukin 15: not so interleukin 2-like after all. J. Exp. Med. 191, 753–756 (2000).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nature Rev. Immunol. 2, 557–568 (2002).
Matsuda, J.L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000).
van der Vliet, H.J. et al. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 100, 144–148 (2001).
Kojo, S., Adachi, Y., Keino, H., Taniguchi, M. & Sumida, T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 44, 1127–1138 (2001).
Fehniger, T.A. & Caligiuri, M.A. Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 (2001).
Husson, H. et al. Functional effects of TNF and lymphotoxin α1β2 on FDC-like cells. Cell. Immunol. 203, 134–143 (2000).
Beutner, U. & MacDonald, H.R. TCR-MHC class II interaction is required for peripheral expansion of CD4 cells in a T cell-deficient host. Int. Immunol. 10, 305–310 (1998).
Viret, C., Wong, F.S. & Janeway, C.A. Jr. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10, 559–568 (1999).
Bender, J., Mitchell, T., Kappler, J. & Marrack, P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J. Exp. Med. 190, 367–374 (1999).
Ernst, B., Lee, D.S., Chang, J.M., Sprent, J. & Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Goldrath, A.W. & Bevan, M.J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190 (1999).
Kieper, W.C. & Jameson, S.C. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA 96, 13306–13311 (1999).
Zeng, D., Lee, M.K., Tung, J., Brendolan, A. & Strober, S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J. Immunol. 164, 5000–5004 (2000).
Ohteki, T., Ho, S., Suzuki, H., Mak, T.W. & Ohashi, P.S. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 159, 5931–5935 (1997).
Kennedy, M.K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Lodolce, J.P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Ohteki, T. et al. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-α/β+ (NK1+T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187, 967–972 (1998).
Walunas, T.L., Wang, B., Wang, C.R. & Leiden, J.M. Cutting edge: the Ets1 transcription factor is required for the development of NK T cells in mice. J. Immunol. 164, 2857–2860 (2000).
Vicari, A.P. et al. NK1.1+ T cells from IL-7-deficient mice have a normal distribution and selection but exhibit impaired cytokine production. Int. Immunol. 8, 1759–1766 (1996).
Gapin, L., Matsuda, J.L., Surh, C.D. & Kronenberg, M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nature Immunol. 2, 971–978 (2001).
Pellicci, D.G. et al. NKT cells develop through a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195, 835–844 (2002).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NKT cell lineage. Science 296, 553–555 (2002).
Kieper, W.C. et al. Over-expression of IL-7 leads to IL-15 independent generation of memory-phenotype CD8+ T cells. J. Exp. Med. 195, 1533–1539 (2002).
Tan, J.T. et al. IL-15 and IL-7 jointly regulate homeostatic proliferation of memory-phenotype CD8+ cells but are not required for memory-phenotype CD4+ cells. J. Exp. Med. 195, 1523–1532 (2002).
Mertsching, E., Burdet, C. & Ceredig, R. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol. 7, 401–414 (1995).
Zhang, X., Sun, S., Hwang, I., Tough, D.F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Coles, M.C. & Raulet, D.H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164, 2412–2418 (2000).
Arase, H., Arase, N. & Saito, T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 183, 2391–2396 (1996).
Eberl, G. & MacDonald, H.R. Rapid death and regeneration of NKT cells in anti-CD3ε- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity 9, 345–353 (1998).
Hammond, K.J. et al. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29, 3768–3781 (1999).
Eberl, G. et al. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162, 6410–6419 (1999).
Carnaud, C. et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 (1999).
Eberl, G., Brawand, P. & MacDonald, H.R. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J. Immunol. 165, 4305–4311 (2000).
Kim, S., Iizuka, K., Aguila, H.L., Weissman, I.L. & Yokoyama, W.M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 97, 2731–2736 (2000).
Becker, T.C. et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195, 1541–1548 (2002).
Goldrath, A.W. et al. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195, 1515–1522 (2002).
Tan, J.T. et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 98, 8732–8737 (2001).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Kawakami, K. et al. Monocyte chemoattractant protein-1-dependent increase of Vα14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J. Immunol. 167, 6525–6532 (2001).
Marrack, P., Kappler, J. & Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–529 (1999).
Hobbs, J.A. et al. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J. Virol. 75, 10746–10754 (2001).
Matsuda, J.L. et al. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor β repertoire and small clone size. Proc. Natl. Acad. Sci. USA 98, 12636–12641 (2001).
von Freeden-Jeffry, U. et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181, 1519–1526 (1995).
Acknowledgements
We thank G. Kim and J. Sprent for helpful discussions, H. Cheroutre for critical reading of the manuscript and TSRI for help with cell sorting. Supported by NIH grants CA52511 (to M. K.) and AI45809 (to C. D. S.) and a grant from the Human Frontiers of Science Research Program (to M. K.). L. G. is supported by the Cancer Research Institute, W. C. K. is supported by U.S. Public Health Service Institutional National Research Service Award AI07244, J. T. T. is supported by US Public Health Service Institute National Research Service Award H07196 and C. D. S. is supported by the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Matsuda, J., Gapin, L., Sidobre, S. et al. Homeostasis of Vα14i NKT cells. Nat Immunol 3, 966–974 (2002). https://doi.org/10.1038/ni837
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni837
This article is cited by
-
Classical MHC expression by DP thymocytes impairs the selection of non-classical MHC restricted innate-like T cells
Nature Communications (2021)
-
Factors that influence the thymic selection of CD8αα intraepithelial lymphocytes
Mucosal Immunology (2021)
-
Modulation of TCR signalling components occurs prior to positive selection and lineage commitment in iNKT cells
Scientific Reports (2021)
-
The role of innate lymphoid cells in response to microbes at mucosal surfaces
Mucosal Immunology (2020)
-
Diversity in medullary thymic epithelial cells controls the activity and availability of iNKT cells
Nature Communications (2020)