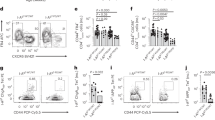
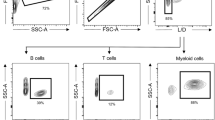
Abstract
Type 1 diabetes is an organ-specific autoimmune disease that is mediated by autoreactive T cells. We show here that administration of a soluble dimeric peptide–major histocompatibility complex (pMHC) class II chimera (DEF) to prediabetic double-transgenic mice prevents the onset of disease or, in animals that are already diabetic, restores normoglycemia. The antidiabetogenic effects of DEF rely on the induction of anergy in splenic autoreactive CD4+ T cells via alteration of early T cell receptor signaling and stimulation of interleukin 10–secreting T regulatory type 1 cells in the pancreas. Soluble dimeric pMHC class II may be useful in the development of immunospecific therapies for type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Buzzetti, R., Quattrocchi, C. C. & Nistico, L. Dissecting the genetics of Type 1 Diabetes: relevance for the familial clustering and differences in incidence. Diabetes Metab. Rev. 14, 111–128 (1998).
Nepon, G. T. & Erlich, H. MHC class-II molecules and autoimmunity. Annu. Rev. Immunol. 9, 493–525 (1991).
Miyakazi, A. et al. Predominance of T lymphocytes in pancreatic islets and spleen of prediabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin. Exp. Immunol. 60, 622–625 (1985).
Like, A. A., Kislaukis, E., Williams, R. R. & Rossini, A. A. Neonatal thymectomy prevents spontaneous diabetes in the BB/W rat. Science 216, 644–646 (1982).
Herold, K. C., Montag, A. G. & Buckingham, F. Induction of tolerance to autoimmune diabetes with islet antigens. J. Exp. Med. 176, 1107–1114 (1992).
Serreze, D. V., Leiter, E. H., Worthen, S. M. & Shultz, L. D. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD X NON)F1 mice. Diabetes 37, 252–255 (1988).
Wicker, L. S., Miller, B. J. & Muller, Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes 35, 855–860 (1986).
Peterson, J. D., Pike, B., McDuffie, M. & Haskins, K. Islet-specific T cell clones transfer diabetes to nonobese diabetic (NOD) F1 mice. J. Immunol. 153, 2800–2806 (1994).
Zekzer, D. et al. Inhibition of diabetes by an insulin-reactive CD4 T-cell clone in the nonobese diabetic mouse. Diabetes 46, 1124–1132 (1997).
Bowman, M. A., Leiter, E. H. & Atkinson, M. A. Prevention of diabetes in the NOD mouse: implications for therapeutic intervention in human disease. Immunol. Today 15, 115–120 (1994).
Verdaguer, J. et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186, 1663–1676 (1997).
Bach, J. F. Insulin dependent diabetes as an autoimmune disease. Endocr. Rev. 15, 516–542 (1994).
Cameron, M. J. et al. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J. Immunol. 159, 4686–4692 (1997).
Nitta, Y. et al. Systemic delivery of interleukin 10 by intramuscular injection of expression plasmid DNA prevents autoimmune diabetes in nonobese diabetic mice. Hum. Gene Ther. 9, 1701–1707 (1998).
Nicoletti, F. et al. Early prophylaxis with recombinant human interleukin-11 prevents spontaneous diabetes in NOD mice. Diabetes 48, 2333–2339 (1999).
Zaccone, P. et al. Interleukin-13 prevents autoimmune diabetes in NOD mice. Diabetes 48, 1522–1528 (1999).
Piccirillo, C. A., Chang, Y. & Prud' homme, G. J. TGF-β1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J. Immunol. 161, 3950–3956 (1998).
Yang, X. D. et al. Effect of tumor necrosis factor α on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J. Exp. Med. 180, 995–1004 (1994).
Herold, K. C. et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes 41, 457–464 (1992).
Wang, Y., Hao, L., Gill, R. G. & Lafferty, K. J. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes 36, 535–538 (1987).
Shizuku, J. A., Taylor-Edwards, C., Banks, B. A., Gregory, A. K. & Fathman, C. G. Immunotherapy of the nonobese mouse: Treatment with an antibody to T-helper lymphocytes. Science 240, 659–662 (1988).
Kuttler, B., Rosing, K., Lehmann, M., Brock, J. & Hahn, H. J. Prevention of autoimmune but not allogeneic destruction of grafted islets by different therapeutic strategies. J. Mol. Med. 77, 226–229 (1999).
Balasa, B. et al. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J. Immunol. 159, 4620–4627 (1997).
Lenschow, D. J. et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 181, 1145–1155 (1995).
Daniel, D. & Wegmann, D. R. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B9-23. Proc. Natl Acad. Sci. USA 93, 956–960 (1996).
Tian, J. et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces TH2 responses and prevents murine insulin-dependent diabetes mellitus. J. Exp. Med. 183, 1561–1567 (1996).
Tian, R. et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs graft survival in diabetes-prone mice. Nature Med. 2, 1384–1353 (1996).
Maron, R., Melican, N. S. & Weiner, H. L. Regulatory TH2-type T cell lines against insulin and GAD peptides derived from orally- and nasally-treated NOD mice suppress diabetes. J. Autoimmun. 12, 251–258 (1999).
Tisch, T., Wang, B. & Serreze, D. V. Induction of glutamic acid decarboxylase 65-specific TH2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J. Immunol. 163, 1178–1187 (1999).
Elias, D. et al. Vaccination against autoimmune mouse diabetes in young NOD mice with a T-cell epitope of the human 65-kDa heat shock protein. Proc. Natl Acad. Sci. USA 88, 3088–3091 (1991).
Bockova, J., Elias, D. & Cohen, I. R. Treatment of NOD diabetes with a novel peptide of the hsp60 molecule induces TH2-type antibodies. J. Autoimmun. 10, 323–329 (1997).
Petersen, J. S. et al. Treatment with GAD65 or BSA does not protect against diabetes in BB rats. Autoimmunity 25, 129–138 (1997).
Funda, D. P., Hartoft-Nielsen, M. L. Kaas, A. & Buschard, K. Effect of intrathymic administration of mycobacterial heat shock protein 65 and peptide p277 on the development of diabetes in NOD mice: caution required in vaccination studies. APMIS 106, 1009–1016 (1998).
Ishioka, G. Y. et al. Failure to demonstrate ling-lived MHC saturation both in vitro and in vivo. Implications for therapeutic potential of MHC-blocking peptides. J. Immunol. 152, 4310–4319 (1994).
Casares, S., Bot, A., Brumeanu, T.-D. & Bona, C. A. Foreign peptides expressed in engineered chimeric self molecules. Biotech. Genet. Engineer. Rev. 15, 159–198 (1998).
Casares, S., Bona, C. A. & Brumeanu, T.-D. Engineering and characterization of a murine MHC-immunoglobulin chimera expressing an immunodominant CD4 T viral epitope. Protein Engineer. 10, 1295–1301 (1997).
Brumeanu, T. D., Bona, C. A. & Casares, S. T cell tolerance and autoimmune diabetes. Int. Rev. Immunol. 20, 301–323 (2001).
Casares, S., Bona, C. A. & Brumeanu, T. D. Enzymatically mediated engineering of multivalent MHC class II-peptide chimeras. Protein Engineer. 14, 195–200 (2001).
Casares, S. et al. Antigen specific signaling by a soluble dimeric peptide-MHC class II-Fc chimera leading to TH2 differentiation. J. Exp. Med. 190, 543–553 (1999).
Hamad, A. R. et al. Potent T cell activation with dimeric peptide-major histocompatibility complex class II ligand: the role of CD4 coreceptor. J. Exp. Med. 188, 1633–1640 (1998).
Appel, H., Gauthier, L., Pyrdol, J. & Wucherpfennig, K. W. Kinetic of T cell receptor binding by bivalent HLA-DR/peptide complexes that activate antigen-specific human T cells. J. Biol. Chem. 275, 312–321 (2000).
Appel, H., Seth, N. P., Gauthier, L. & Wucherpfennig, K. W. Anergy induction by dimeric TCR ligands. J. Immunol. 166, 5279–5285 (2001).
Radu, D., Brumeanu, T.-D., McEvoy, R. C., Bona, C. A. & Casares, S. Escape from natural self-tolerance leads to neonatal insulin-dependent diabetes mellitus. Autoimmunity 30, 199–207 (1999).
Sarukhan, A. et al. Changes in function of antigen-specific lymphocytes correlating with progression towards diabetes in a transgenic model. EMBO J. 17, 71–80 (1998).
Scott, B. et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity 1, 73–83 (1994).
Lo, D. et al. Peripheral tolerance to an islet cell-specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur. J. Immunol. 22, 1013–1022 (1992).
Bot, A., Casares, S., Bot, S., von Boehmer, H. & Bona, C. A. Cellular mechanisms involved in protection against influenza virus infection in transgenic mice expressing a TCR specific for class-II hemagglutinin peptide in CD4+ and CD8+ T cells. J. Immunol. 160, 4500–4507 (1998).
Sarukhan, A., Garcia, C., Lanoue, A. & von Boehmer, H. Allelic inclusion of T cell receptor alpha genes poses an autoimmune hazard due to low-level expression of autospecific receptors. Immunity 6, 563–570 (1998).
Levings, M. K. & Roncarolo, M. G. T-regulatory 1 cells: a novel subset of CD4 T cells with immunomodulatory properties. J. Allergy Clin. Immunol. 106, 109–112 (2000).
Rabinovitch, A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 14, 129–151 (1998).
Ravetch, J. V. & Bolland, S. IgG Fc receptors. Ann. Rev. Immunol. 19, 275–290 (2001).
Weil, R. & Veillette, A. Signal transduction by the lymphocyte-specific tyrosine protein kinase p56lck. Curr. Top. Microbiol. Immunol. 205, 63–87 (1996).
La Face, D. M. et al. Differential T cell signaling induced by antagonist peptide-MHC complexes and the associated phenotypic responses. J. Immunol. 158, 2057–2064 (1997).
Faith, A. et al. An altered peptide ligand inhibits TH2 cytokine synthesis by abrogating TCR signaling. J. Immunol. 162, 1836–1842 (1999).
Migita, K. et al. Defective TCR-mediated signaling in anergic T cells. J. Immunol. 155, 5083–5087 (1995).
Madrenas, J., Chau, L. A., Smith, J., Bluestone, J. A. & Germain, R. N. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J. Exp. Med. 185, 219–229 (1997).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Frey, M. et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J. Immunol, 161, 400–408 (1998).
Bruunsgaard, H., Pedersen, A. N., Schroll, M., Skinhoj, P. & Pedersen, B. K. Proliferative responses of blood mononuclear cells (PBMC) in a cohort of elderly humans: role of lymphocyte phenotype and cytokine production. Clin. Exp. Immunol. 119, 433–440 (2000).
Lepault, F. & Gagnerault, M. C. Characterization of peripheral regulatory CD4 T cells that prevent diabetes onset in nonobese diabetic mice. J. Immunol, 164, 240–247 (2000).
Seddon, B., Saoudi, A., Nicholson, M. & Mason, D. CD4+CD8− thymocytes that express L-selectin protect rats from diabetes upon adoptive transfer. Eur. J. Immunol. 26, 2702–2708 (1996).
Alleva, D. G. et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J. Clin. Invest. 107, 173–180 (2001).
Wicker, L. S. et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele DRB1*0401. J. Clin. Invest. 98, 2597–2603 (1996).
Endl, J. et al. Identification of naturally processed T cells epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J. Clin. Invest. 99, 2405–2415 (1997).
Patel, S. D. et al. Identification of immunodominant T cell epitopes of human glutamic acid decarboxylase 65 by using HLA–DR(α1*0101,β1*0401) transgenic mice. Proc. Natl Acad. Sci. USA 94, 8082–8087 (1997).
Acknowledgements
We thank A. Miller for helpful discussions. Supported by grants from the Juvenile Diabetes Foundation International, Alexander & Alexandrine L. Sinsheimer Foundation and from the National Institutes of Health (grant 1R55DK55744 to S. C. and grants 1R01DK061927 and 1R21DK61326 to T.-D. B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Casares, S., Hurtado, A., McEvoy, R. et al. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide–MHC class II chimera. Nat Immunol 3, 383–391 (2002). https://doi.org/10.1038/ni770
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni770
This article is cited by
-
Antigen-specific therapeutic approaches for autoimmunity
Nature Biotechnology (2019)
-
Increased yields and biological potency of knob-into-hole-based soluble MHC class II molecules
Nature Communications (2019)
-
Peptide-MHC-based nanovaccines for the treatment of autoimmunity: a “one size fits all” approach?
Journal of Molecular Medicine (2011)
-
IL-2 as a therapeutic target for the restoration of Foxp3+ regulatory T cell function in organ-specific autoimmunity: implications in pathophysiology and translation to human disease
Journal of Translational Medicine (2010)
-
Multimer technologies for detection and adoptive transfer of antigen-specific T cells
Cancer Immunology, Immunotherapy (2010)