Abstract
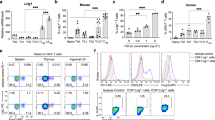
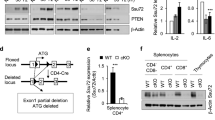
Stra13, a basic helix-loop-helix transcription factor, is up-regulated upon activation of CD4+ T cells. Here we show that Stra13-deficient mice exhibit defects in several phases of CD4+ T cell activation. In vivo, Stra13 deficiency results in ineffective elimination of activated T and B cells, which accumulate progressively, leading to lymphoid organ hyperplasia. Consequently, aging Stra13−/− mice develop autoimmune disease characterized by accumulation of spontaneously activated T and B cells, circulating autoantibodies, infiltration of T and B lymphocytes in several organs and immune complex deposition in glomeruli. Our studies identify Stra13 as a key regulator of lymphocyte activation that is vital for maintenance of self-tolerance and for constraint of autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Mondino, A., Khoruts, A. & Jenkins, M. K. The anatomy of T-cell activation and tolerance. Proc. Natl Acad. Sci. USA 93, 2245–2252 (1996).
Jenkins, M. K. et al. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19, 23–45 (2001).
Abbas, A. K., Murphy, K. M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Boudjelal, M. et al. Overexpression of Stra13, a novel retinoic acid–inducible gene of the basic helix-loop-helix family, inhibits mesodermal and promotes neuronal differentiation of P19 cells. Genes Dev. 11, 2052–2065 (1997).
Ishibashi, M. et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 9, 3136–3148 (1995).
Tomita, K. et al. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev. 13, 1203–1210 (1999).
Sun, H. & Taneja, R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc. Natl Acad. Sci. USA 97, 4058–4063 (2000).
Smith, K. A. Interleukin-2: inception, impact, and implications. Science 240, 1169–1176 (1988).
Ye, B. H. et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nature Genet. 16, 161–170 (1997).
Dent, A. L., Shafer, A. L., Yu, X., Allman, D. & Staudt, L. M. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276, 589–592 (1997).
Rose, M. L., Birbeck, M. S., Waliis, V. J., Forrester, J. A. & Davies, A. J. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature 284, 364–366 (1980).
Watanabe-Fukunaga, R., Brannan, C. I., Copeland, N. G., Jenkins, N. A. & Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992).
Takahashi, T. et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76, 969–976 (1994).
Gorelik, L. & Flavell, R.A. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12,171–181 (2000).
Abbas, A. K. Die and let live: eliminating dangerous lymphocytes. Cell 84, 655–657 (1996).
Rathmell, J. C., Townsend, S. E., Xu, J. C., Flavell, R. A. & Goodnow, C. C. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell 87, 319–329 (1996).
Alderson, M. R. et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 181, 71–77 (1995).
Oberg, H. H., Lengl-Janssen, B., Kabelitz, D. & Janssen, Q. Activation-induced T cell death: resistance or susceptibility correlate with cell surface fas ligand expression and T helper phenotype. Cell Immunol. 181, 93–100 (1997).
Sadlack, B. et al. Ulcerative colitis–like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993).
Sadlack, B. et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25, 3053–3059 (1995).
Willerford, D. M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476. (1995).
Noble, A. Review article: molecular signals and genetic reprogramming in peripheral T-cell differentiation. Immunology 101, 289–299 (2000).
Dong, C. & Flavell, R. A. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2, 179–188 (2000).
Kuo, C. T. & Leiden, J. M. Transcriptional regulation of T lymphocyte development and function. Annu. Rev. Immunol. 17, 149–187 (1999).
Glimcher, L. H. & Murphy, K. M. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14, 1693–1711 (2000).
Serfling, E. et al. The role of NF-AT transcription factors in T cell activation and differentiation (1). Biochim. Biophys. Acta. 1498, 1–18 (2000).
Peng, S. L., Gerth, A. J., Ranger, A. M. & Glimcher, L. H. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 14, 13–20 (2001).
Gerondakis, S., Grumont, R., Rourke, I. & Grossmann, M. The regulation and roles of Rel/NK-κB transcription factors during lymphocyte activation. Curr. Opin. Immunol. 10, 253–359 (1998).
Kasibhatla, S. et al. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol. Cell 1, 543–551 (1998).
Rooney, J. W., Hoey, T. & Glimcher, L. H. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the murine IL-4 gene. Immunity 2, 473–483 (1995).
Fisher, G. H. et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81, 935–946 (1995).
Rieux-Laucat, F. et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268, 1347–1351 (1995).
Refaeli, Y., Van Parijs, L., London, C.A., Tschopp, J. & Abbas, A.K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8, 615–623 (1998).
Van Parijs, L. et al. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11, 281–288 (1999).
Dhar, H. & Taneja, R. Cross regulatory interaction between Stra13 and USF results in functional antagonism. Oncogene 20, 4750–4756 (2001).
Sun, H., Mattei, M. G. & Taneja, R. Assignment of Stra13 to the sub-telomeric region of mouse chromosome 6 by in situ hybridization. Cytogenet. Cell Genet. 87, 211–212 (1999).
Acknowledgements
We thank G. Cattoretti for advice on histopathology, M. Mendelsohn for blastocyst injections, P. Chambon for Stra13 antibodies and E. Eynon and D. Hesslein for advice and reagents. We are grateful to K. Calame, G. Cattoretti and J. Bieker for comments on the manuscript. B.L. is an Associate and R.A.F. is an Investigator of the Howard Hughes Medical Institute. R.T. is a Basil O'Connor Research Scholar. Supported in part by grants from the NIH, March of Dimes, Waxman Cancer Research Foundation and Charlotte Geyer Foundation (to R.T.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Web Figure 1.
Flow cytometric analysis of thymocytes from 8-week old wild-type and Stra13-deficient mice. Thymocytes were stained with various cell surface markers as indicated. (GIF 61 kb)
Web Figure 2.
Flow cytometric analysis of bone marrow cells from 8-week-old wild-type and Stra13-deficient mice. Cells were stained with lineage specific markers as indicated. (GIF 66 kb)
Web Figure 3.
KLH-specific primary antibody responses. Wild-type (filled circle) and Stra13-/- (open circle) mice were immunized with KLH in CFA, and bled on day 14 after boosting. KLH-specific Ig was analyzed by ELISA. (GIF 25 kb)
Web Figure 4.
Analysis of B cell clonality by PCR. DNA samples prepared from spleen of 8-month old wild-type (+/+) and Stra13-/- mice were analyzed for DH-JH rearrangement. The predicted rearrangements using various J segments are shown. The expected size of JH1, JH2 and JH3 are 1.2, 0.7 and 0.3-0.4 kb. (GIF 40 kb)
Web Figure 5.
Analysis of B1 B cells in 8-month old wild-type and Stra13-deficient mice. Single cell suspension from spleen and peritoneum were double-stained with anti-IgM and anti-CD5 and analyzed by flow cytometric analysis. (GIF 58 kb)
Rights and permissions
About this article
Cite this article
Sun, H., Lu, B., Li, RQ. et al. Defective T cell activation and autoimmune disorder in Stra13-deficient mice. Nat Immunol 2, 1040–1047 (2001). https://doi.org/10.1038/ni721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni721