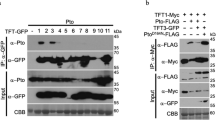
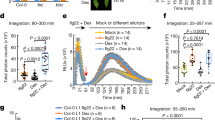
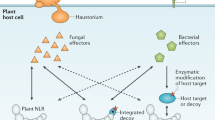
Abstract
Plant proteins belonging to the nucleotide-binding site–leucine-rich repeat (NBS-LRR) family are used for pathogen detection. Like the mammalian Nod-LRR protein 'sensors' that detect intracellular conserved pathogen-associated molecular patterns, plant NBS-LRR proteins detect pathogen-associated proteins, most often the effector molecules of pathogens responsible for virulence. Many virulence proteins are detected indirectly by plant NBS-LRR proteins from modifications the virulence proteins inflict on host target proteins. However, some NBS-LRR proteins directly bind pathogen proteins. Association with either a modified host protein or a pathogen protein leads to conformational changes in the amino-terminal and LRR domains of plant NBS-LRR proteins. Such conformational alterations are thought to promote the exchange of ADP for ATP by the NBS domain, which activates 'downstream' signaling, by an unknown mechanism, leading to pathogen resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chisholm, S.T., Coaker, G., Day, B. & Staskawicz, B.J. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006).
Ausubel, F.M. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6, 973–979 (2005).
Inohara. Chamaillard, McDonald, C. & Nunez, G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74, 355–383 (2005).
Pan, Q., Wendel, J. & Fluhr, R. Divergent evolution of plant NBS-LRR resistance gene homologues in dicot and cereal genomes. J. Mol. Evol. 50, 203–213 (2000).
van der Biezen, E.A. & Jones, J.D.G. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem. Sci. 23, 454–456 (1998).
Jia, Y., McAdams, S.A., Bryan, G.T., Hershey, H.P. & Valent, B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014 (2000).
Bryan, G.T. et al. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12, 2033–2046 (2000).
Deslandes, L. et al. Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA 100, 8024–8029 (2003).
Deslandes, L. et al. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proc. Natl. Acad. Sci. USA 99, 2404–2409 (2002).
Dodds, P.N. et al. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA 103, 8888–8893 (2006).
Grant, M.R. et al. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843–846 (1995).
Innes, R.W., Bent, A.F., Kunkel, B.N., Bisgrove, S.R. & Staskawicz, B.J. Molecular analysis of avirulence gene avrRpt2 and identification of a putative regulatory sequence common to all known Pseudomonas syringae avirulence genes. J. Bacteriol. 175, 4859–4869 (1993).
Bent, A.F. et al. RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265, 1856–1860 (1994).
Mindrinos, M., Katagiri, F., Yu, G.L. & Ausubel, F.M. The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78, 1089–1099 (1994).
Axtell, M.J. & Staskawicz, B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377 (2003).
Mackey, D., Holt, B.F.I., Wiig, A. & Dangl, J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108, 743–754 (2002).
Coaker, G., Falick, A. & Staskawicz, B. Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science 308, 548–550 (2005).
Day, B. et al. Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell 17, 1292–1305 (2005).
Kim, H.S. et al. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc. Natl. Acad. Sci. USA 102, 6496–6501 (2005).
Belkhadir, Y., Nimchuk, Z., Hubert, D.A., Mackey, D. & Dangl, J.L. Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. Plant Cell 16, 2822–2835 (2004).
Lim, M.T. & Kunkel, B.N. The Pseudomonas syringae type III effector AvrRpt2 promotes virulence independently of RIN4, a predicted virulence target in Arabidopsis thaliana. Plant J. 40, 790–798 (2004).
Simonich, M.T. & Innes, R.W. A disease resistance gene in Arabidopsis with specificity for the avrPph3 gene of Pseudomonas syringae pv. phaseolicola. Mol. Plant Microbe Interact. 8, 637–640 (1995).
Warren, R.F., Henk, A., Mowery, P., Holub, E. & Innes, R.W. A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10, 1439–1452 (1998).
Swiderski, M.R. & Innes, R.W. The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J. 26, 101–112 (2001).
Shao, F. et al. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301, 1230–1233 (2003).
Shao, F., Merritt, P.M., Bao, Z., Innes, R.W. & Dixon, J.E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109, 575–588 (2002).
Salmeron, J.M., Barker, S.J., Carland, F.M., Mehta, A.Y. & Staskawicz, B.J. Tomato mutants altered in bacterial disease resistance provide evidence for a new locus controlling pathogen recognition. Plant Cell 6, 511–520 (1994).
Salmeron, J.M. et al. Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86, 123–133 (1996).
Pedley, K.F. & Martin, G.B. Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu. Rev. Phytopathol. 41, 215–243 (2003).
Martin, G.B. et al. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262, 1432–1436 (1993).
Scofield, S.R. et al. Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274, 2063–2065 (1996).
Tang, X. et al. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274, 2060–2063 (1996).
Mucyn, T.S. et al. The NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell advance online publication, 6 October 2006 (doi: 10.1105/tpc.106.044016).
Kobe, B. & Deisenhofer, J. The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19, 415–421 (1994).
Michelmore, R.W. & Meyers, B.C. Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 8, 1113–1130 (1998).
Ellis, J.G., Lawrence, G.J., Luck, J.E. & Dodds, P.N. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11, 495–506 (1999).
Luck, J.E., Lawrence, G.J., Dodds, P.N., Shepherd, K.W. & Ellis, J.G. Regions outside of the leucine-rich repeats of flax rust resistance proteins play a role in specificity determination. Plant Cell 12, 1367–1377 (2000).
Tao, Y., Yuan, F., Leister, R.T., Ausubel, F.M. & Katagiri, F. Mutational analysis of the Arabidopsis nucleotide binding site-leucine-rich repeat resistance gene RPS2. Plant Cell 12, 2541–2554 (2000).
Weaver, M.L., Swiderski, M.R., Li, Y. & Jones, J.D.G. The Arabidopsis thaliana TIR-NB-LRR R-protein, RPP1A; protein localization and constitutive activation of defence by truncated alleles in tobacco and Arabidopsis. Plant J. 47, 829–840 (2006).
Bendahmane, A., Farnham, G., Moffett, P. & Baulcombe, D.C. Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 32, 195–204 (2002).
Tanabe, T. et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23, 1587–1597 (2004).
Inohara, N. et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J. Biol. Chem. 274, 14560–14567 (1999).
Rairdan, G.J. & Moffett, P. Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. Plant Cell 18, 2082–2093 (2006).
Hwang, C.F., Bhakta, A.V., Truesdell, G.M., Pudlo, W.M. & Williamson, V.M. Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell 12, 1319–1329 (2000).
Hwang, C.F. & Williamson, V.M. Leucine-rich repeat-mediated intramolecular interactions in nematode recognition and cell death signaling by the tomato resistance protein Mi. Plant J. 34, 585–593 (2003).
Moffett, P., Farnham, G., Peart, J. & Baulcombe, D.C. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 21, 4511–4519 (2002).
Tameling, W.I. et al. Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol. 140, 1233–1245 (2006).
Lawrence, G.J., Finnegan, E.J., Ayliffe, M.A. & Ellis, J.G. The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N. Plant Cell 7, 1195–1206 (1995).
Anderson, P.A. et al. Inactivation of the flax rust resistance gene M associated with loss of a repeated unit within the leucine-rich repeat coding region. Plant Cell 9, 641–651 (1997).
Parker, J.E. et al. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 9, 879–894 (1997).
Ayliffe, M.A. et al. Analysis of alternative transcripts of the flax L6 rust resistance gene. Plant J. 17, 287–292 (1999).
Dinesh-Kumar, S.P. & Baker, B.J. Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc. Natl. Acad. Sci. USA 97, 1908–1913 (2000).
van der Biezen, E.A. & Jones, J.D.G. The NB-ARC domain: a novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr. Biol. 8, R226–R227 (1998).
Takken, F.L., Albrecht, M. & Tameling, W.I. Resistance proteins: molecular switches of plant defence. Curr. Opin. Plant Biol. 9, 383–390 (2006).
Meyers, B.C. et al. Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J. 20, 317–332 (1999).
Meyers, B.C., Kozik, A., Griego, A., Kuang, H. & Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15, 809–834 (2003).
Shirano, Y., Kachroo, P., Shah, J. & Klessig, D.F. A gain-of-function mutation in an Arabidopsis Toll interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 14, 3149–3162 (2002).
de la Fuente van Bentem, S. et al. Heat shock protein 90 and its co-chaperone protein phosphatase 5 interact with distinct regions of the tomato I-2 disease resistance protein. Plant J. 43, 284–298 (2005).
Tornero, P., Chao, R.A., Luthin, W.N., Goff, S.A. & Dangl, J.L. Large-scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 14, 435–450 (2002).
Howles, P. et al. Autoactive alleles of the flax L6 rust resistance gene induce non-race-specific rust resistance associated with the hypersensitive response. Mol. Plant Microbe Interact. 18, 570–582 (2005).
Riedl, S.J., Li, W., Chao, Y., Schwarzenbacher, R. & Shi, Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 434, 926–933 (2005).
Yan, N. et al. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature 437, 831–837 (2005).
Albrecht, M. & Takken, F.L. Update on the domain architectures of NLRs and R proteins. Biochem. Biophys. Res. Commun. 339, 459–462 (2006).
Hu, Y., Benedict, M.A., Ding, L. & Nunez, G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 18, 3586–3595 (1999).
Jiang, X. & Wang, X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J. Biol. Chem. 275, 31199–31203 (2000).
Tameling, W.I. et al. The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell 14, 2929–2939 (2002).
Sprang, S.R. G protein mechanisms: insights from structural analysis. Annu. Rev. Biochem. 66, 639–678 (1997).
Schreiber, S., Rosenstiel, P., Albrecht, M., Hampe, J. & Krawczak, M. Genetics of Crohn disease, an archetypal inflammatory barrier disease. Nat. Rev. Genet. 6, 376–388 (2005).
Aarts, N. et al. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 10306–10311 (1998).
Tao, X., Xu, Y., Zheng, Y., Beg, A.A. & Tong, L. An extensively associated dimer in the structure of the C713S mutant of the TIR domain of human TLR2. Biochem. Biophys. Res. Commun. 299, 216–221 (2002).
Xu, Y. et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000).
Mestre, P. & Baulcombe, D.C. Elicitor-mediated oligomerization of the tobacco N disease resistance protein. Plant Cell 18, 491–501 (2006).
Leister, R.T. et al. Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana. Plant Cell 17, 1268–1278 (2005).
Leister, D. et al. Rapid reorganization of resistance gene homologues in cereal genomes. Proc. Natl. Acad. Sci. USA 95, 370–375 (1998).
Zhang, Y., Goritschnig, S., Dong, X. & Li, X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1–1, constitutive 1. Plant Cell 15, 2636–2646 (2003).
Pettersen, E.F. et al. UCSF Chimera — a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank J. Rathjen for sharing a manuscript in the press. Supported by the US National Institutes of Health (GM046451 to R.W.I.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
DeYoung, B., Innes, R. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7, 1243–1249 (2006). https://doi.org/10.1038/ni1410
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1410
This article is cited by
-
Genome-wide identification and characterization of NBLRR genes in finger millet (Eleusine coracana L.) and their expression in response to Magnaporthe grisea infection
BMC Plant Biology (2024)
-
Panicle transcriptome of high-yield mutant indica rice reveals physiological mechanisms and novel candidate regulatory genes for yield under reproductive stage drought stress
BMC Plant Biology (2023)
-
MG1 interacts with a protease inhibitor and confers resistance to rice root-knot nematode
Nature Communications (2023)
-
Genome-Wide Identification, Structural Diversity Analysis and Expression Profiling of NBARC Domain-Containing Gene Family in Pisum sativum
National Academy Science Letters (2023)
-
Podosphaera cerasi- an old foe of US sweet cherry with a new name –its biology, epidemiology, and beyond
Journal of Plant Pathology (2023)