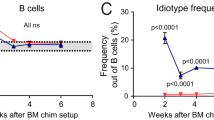
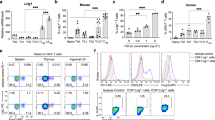
Abstract
T cells expressing an invariant Vα19-Jα33 T cell receptor α-chain (Vα19i TCR) are restricted by the nonpolymorphic major histocompatibility complex class Ib molecule MR1. Whether Vα19i T cells are involved in autoimmunity is not understood. Here we demonstrate that T cells expressing the Vα19i TCR transgene inhibited the induction and progression of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. Similarly, EAE was exacerbated in MR1-deficient mice, which lack Vα19i T cells. EAE suppression was accompanied by reduced production of inflammatory mediators and increased secretion of interleukin 10. Interleukin 10 production occurred at least in part through interactions between B cells and Vα19i T cells mediated by the ICOS costimulatory molecule. These results suggest an immunoregulatory function for Vα19i T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradox. Annu. Rev. Immunol. 23, 877–900 (2005).
Treiner, E. et al. Mucosal-associated invariant T (MAIT) cells: an evolutionarily conserved T cell subset. Microbes Infect. 7, 552–559 (2005).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Okamoto, N. et al. Synthetic α-mannosyl ceramide as a potent stimulant for an NKT cell repertoire bearing the invariant Vα19-Jα26 TCR α chain. Chem. Biol. 12, 677–683 (2005).
Chen, Y.H., Chiu, N.M., Mandal, M., Wang, N. & Wang, C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity 6, 459–467 (1997).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Spada, F.M., Koezuka, Y. & Porcelli, S.A. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188, 1529–1534 (1998).
Tilloy, F. et al. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted α/β T cell subpopulation in mammals. J. Exp. Med. 189, 1907–1921 (1999).
Godfrey, D.I., MacDonald, H.R., Kronenberg, M., Smyth, M.J. & Van Kaer, L. NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
Lehuen, A. et al. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 188, 1831–1839 (1998).
Mars, L.T. et al. Vα14-Jα281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J. Immunol. 168, 6007–6011 (2002).
Wagner, M.J., Hussain, S., Mehan, M., Verdi, J.M. & Delovtch, T.L. A defect in lineage fate decision during fetal thymic invariant NKT cell development may regulate susceptibility to type 1 diabetes. J. Immunol. 174, 6764–6771 (2005).
Pál, E. et al. Costimulation-dependent modulation of experimental autoimmune encephalomyelitis by ligand stimulation of Vα14 NK T cells. J. Immunol. 166, 662–668 (2001).
Sharif, S. et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat. Med. 7, 1057–1062 (2001).
Hong, S. et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7, 1052–1056 (2001).
Miyamoto, K., Miyake, S. & Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001).
Chiba, A. et al. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of α-galactosylceramide. Arthritis Rheum. 50, 305–313 (2004).
Miyake, S. & Yamamura, T. Therapeutic potential of glycolipid ligands for natural killer (NK) T cells in the suppression of autoimmune diseases. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5, 315–322 (2005).
Chiba, A., Kaieda, S., Oki, S., Yamamura, T. & Miyake, S. The involvement of Vα14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 52, 1941–1948 (2005).
Kim, H.Y. et al. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor β1 production. J. Exp. Med. 201, 41–47 (2005).
Porcelli, S., Yockey, C.E., Brenner, M.B. & Balk, S.P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 178, 1–16 (1993).
Shimamura, M. & Huang, Y.Y. Presence of a novel subset of NKT cells bearing an invariant Vα19.1-Jα26 TCR α chain. FEBS Lett. 516, 97–100 (2002).
Kawachi, I., Maldonado, J., Strader, C. & Gilfillan, S. MR1-restricted Vα19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J. Immunol. 176, 1618–1627 (2006).
Illés, Z., Shimamura, M., Newcombe, J., Oka, N. & Yamamura, T. Accumulation of Vα7.2-Jα33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int. Immunol. 16, 223–230 (2004).
Illés, Z. et al. Differential expression of NK T cell Vα24JαQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 164, 4375–4381 (2000).
Croxford, J.L., Feldmann, M., Chernajovsky, Y. & Baker, D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependent upon the mode of delivery of IL-10: a comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J. Immunol. 166, 4124–4130 (2001).
Croxford, J.L. et al. Cytokine gene therapy in experimental allergic encephalomyelitis by injection of plasmid DNA-cationic liposome complex into the central nervous system. J. Immunol. 160, 5181–5187 (1998).
Cua, D.J., Groux, H., Hinton, D.R., Stohlman, S.A. & Coffman, R.L. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 189, 1005–1010 (1999).
Bettelli, E., Nicholson, L.B. & Kuchroo, V.K. IL-10, a key effector regulatory cytokine in experimental autoimmune encephalomyelitis. J. Autoimmun. 20, 265–267 (2003).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Fillatreau, S., Sweenie, C.H., McGeachy, M.J., Gray, D. & Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002).
Mauri, C., Gray, D., Mushtaq, N. & Londei, M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 197, 489–501 (2003).
Riegert, P., Wanner, V. & Bahram, S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J. Immunol. 161, 4066–4077 (1998).
Hayakawa, Y. et al. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166, 6012–6018 (2001).
Ikarashi, Y. et al. Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: critical role for B7 in CD1d-dependent NKT cell interferon γ production. J. Exp. Med. 194, 1179–1186 (2001).
Kitamura, H. et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1128 (1999).
Kaneda, H. et al. ICOS costimulates invariant NKT cell activation. Biochem. Biophys. Res. Commun. 327, 201–207 (2005).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
McAdam, A.J. et al. ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 (2001).
Song, F. et al. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J. Immunol. 162, 2275–2280 (1999).
Kaneko, M., Akiyama, Y., Takimoto, H. & Kumazawa, Y. Mechanism of up-regulation of immunoglobulin A production in the intestine of mice unresponsive to lipopolysaccharide. Immunology 116, 64–70 (2005).
Cognasse, F. et al. Differential downstream effects of CD40 ligation mediated by membrane or soluble CD40L and agonistic Ab: a study on purified human B cells. Int. J. Immunopathol. Pharmacol. 18, 65–74 (2005).
Muhlen, K.A. et al. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J. Immunol. 172, 3034–3041 (2004).
Acknowledgements
We thank S. Gilfillan (Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri) for Mr1−/− mice. Supported by the Japan Society for the Promotion of Science (P03581 to J.L.C), the Ministry of Health, Labour and Welfare of Japan (T.Y. and S.M.), The Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (02-5 to T.Y.), Grant-in-Aid for Science Research on Priority Area from Ministry of Education, Science, Sports and Culture of Japan (17047051 to S.M.) and Grant-in-Aid for Scientific Research (B) (18390295 to S.M.) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression of adhesion molecules on the surface of TCRβ+ T cells from Vα19iTgCd1d−/− mice is inhibited during EAE. (PDF 307 kb)
Supplementary Fig. 2
MOG35-55 peptide enhances Vα19i T cell-mediated IL-10 production. (PDF 255 kb)
Supplementary Table 1
Table shows primer gene names and sequences (5′ to 3′) used in this paper for real-time RT-PCR. (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Croxford, J., Miyake, S., Huang, YY. et al. Invariant Vα19i T cells regulate autoimmune inflammation. Nat Immunol 7, 987–994 (2006). https://doi.org/10.1038/ni1370
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1370
This article is cited by
-
Mucosal-associated invariant T cells in infectious diseases of respiratory system: recent advancements and applications
Journal of Inflammation (2024)
-
Mucosal-associated invariant T cells have therapeutic potential against ocular autoimmunity
Mucosal Immunology (2022)
-
The immunology of multiple sclerosis
Nature Reviews Immunology (2022)
-
Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals
Nature Communications (2021)
-
MAIT cells, guardians of skin and mucosa?
Mucosal Immunology (2021)