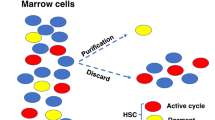
Abstract
A signature characteristic of stem cells is their ability to self-renew, affording a theoretically limitless ability to produce daughter cells and their descendents. This near-timeless dimension of stem cell function is not free of the constraints of place. The idea that highly specialized 'microenvironmental' cues participate in the regulation of stem cells has evidence in classic embryology and more recently in adult stem cells through the use of model organisms. There is now ample evidence that an anatomically defined, specifically constituted place represents the niche for hematopoietic and other tissue-specific stem cells. This review provides a conceptual framework and detailed account of the hematopoietic stem cell niche as defined at present. The components are assembling into a more complex view of the niche and may now be amenable to examination as a system and possibly to alteration to affect outcomes in immune regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Xie, T. & Spradling, A.C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Kiger, A.A., Jones, D.L., Schulz, C., Rogers, M.B. & Fuller, M.T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Crittenden, S.L. et al. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417, 660–663 (2002).
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978).
Xie, T. & Spradling, A.C. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 (1998).
Abkowitz, J.L., Catlin, S.N., McCallie, M.T. & Guttorp, P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood 100, 2665–2667 (2002).
Song, X., Zhu, C.H., Doan, C. & Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857 (2002).
Yamashita, Y.M., Jones, D.L. & Fuller, M.T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550 (2003).
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004).
Decotto, E. & Spradling, A.C. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9, 501–510 (2005).
Crittenden, S.L. et al. Regulation of the mitosis/meiosis decision in the Caenorhabditis elegans germline. Phil. Trans. R. Soc. Lond. B 358, 1359–1362 (2003).
Calvi, L.M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Kai, T. & Spradling, A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl. Acad. Sci. USA 100, 4633–4638 (2003).
Kai, T. & Spradling, A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428, 564–569 (2004).
Palis, J., Robertson, S., Kennedy, M., Wall, C. & Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073–5084 (1999).
Muller, A.M., Medvinsky, A., Strouboulis, J., Grosveld, F. & Dzierzak, E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301 (1994).
Gekas, C., Dieterlen-Lievre, F., Orkin, S.H. & Mikkola, H.K. The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365–375 (2005).
Wright, D.E., Wagers, A.J., Gulati, A.P., Johnson, F.L. & Weissman, I.L. Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001).
Frimberger, A.E. et al. The fleet feet of haematopoietic stem cells: rapid motility, interaction and proteopodia. Br. J. Haematol. 112, 644–654 (2001).
Udani, V.M. et al. Hematopoietic stem cells give rise to perivascular endothelial-like cells during brain tumor angiogenesis. Stem Cells Dev. 14, 478–486 (2005).
Stewart, F.M., Crittenden, R.B., Lowry, P.A., Pearson-White, S. & Quesenberry, P.J. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood 81, 2566–2571 (1993).
Nilsson, S.K., Johnston, H.M. & Coverdale, J.A. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 97, 2293–2299 (2001).
Taichman, R.S. & Emerson, S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 179, 1677–1682 (1994).
Taichman, R., Reilly, M., Verma, R., Ehrenman, K. & Emerson, S. Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. Br. J. Haematol. 112, 438–448 (2001).
Mancini, S.J. et al. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 105, 2340–2342 (2005).
Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004).
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006).
Semerad, C.L. et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 106, 3020–3027 (2005).
Wilson, A. et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18, 2747–2763 (2004).
Denhardt, D.T., Noda, M., O'Regan, A.W., Pavlin, D. & Berman, J.S. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 107, 1055–1061 (2001).
Stier, S. et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201, 1781–1791 (2005).
Nilsson, S.K. et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232–1239 (2005).
Silver, I.A., Murrills, R.J. & Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 175, 266–276 (1988).
Chattopadhyay, N., Vassilev, P.M. & Brown, E.M. Calcium-sensing receptor: roles in and beyond systemic calcium homeostasis. Biol. Chem. 378, 759–768 (1997).
Adams, G.B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2005).
Deguchi, K. et al. Excessive extramedullary hematopoiesis in Cbfa1-deficient mice with a congenital lack of bone marrow. Biochem. Biophys. Res. Commun. 255, 352–359 (1999).
Yoshida, H. et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 (1990).
Kiel, M.J., Yilmaz, O.H., Iwashita, T., Terhorst, C. & Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Avecilla, S.T. et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10, 64–71 (2004).
Sipkins, D.A. et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005).
Acknowledgements
We thank C. Shambaugh for administrative assistance. Supported in part by the National Institutes of Health (HL081030 and HL44851), the Burroughs Wellcome Fund and the Leukemia & Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.T.S. is a founder of Zhealix, a company targeting stem cells pharmacologically.
Rights and permissions
About this article
Cite this article
Adams, G., Scadden, D. The hematopoietic stem cell in its place. Nat Immunol 7, 333–337 (2006). https://doi.org/10.1038/ni1331
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1331
This article is cited by
-
Growth dynamics of breast cancer stem cells: effects of self-feedback and EMT mechanisms
Theory in Biosciences (2022)
-
MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies
Journal of Hematology & Oncology (2021)
-
Putative stem cells in the hemolymph and in the intestinal submucosa of the solitary ascidian Styela plicata
EvoDevo (2019)
-
Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization
Leukemia (2019)
-
N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer
BMC Cancer (2018)