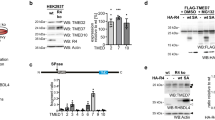
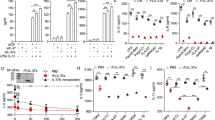
Abstract
Tumor necrosis factor receptor–associated factor 6 (TRAF6) is critical for mediating Toll-like receptor (TLR)–interleukin 1 receptor (IL-1R) signaling and subsequent activation of NF-κB and AP-1, transcriptional activators of innate immunity. Here we show that β-arrestins, a family of multifunctional proteins, directly interacted with TRAF6 after TLR–IL-1R activation. Formation of the β-arrestin–TRAF6 complex prevented autoubiquitination of TRAF6 and activation of NF-κB and AP-1. Endotoxin-treated β-arrestin 2–deficient mice had higher expression of proinflammatory cytokines and were more susceptible to endotoxic shock. Thus, β-arrestins are essential negative regulators of innate immune activation via TLR–IL-1R signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Dunne, A. & O'Neill, L.A. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE 2003, 3–17 (2003).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Bradley, J.R. & Pober, J.S. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20, 6482–6491 (2001).
Chung, J.Y., Park, Y.C., Ye, H. & Wu, H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115, 679–688 (2002).
Lomaga, M.A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Deng, L. et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 (2000).
Wang, C. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Kovalenko, A. et al. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature 424, 801–805 (2003).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424, 793–796 (2003).
Brummelkamp, T.R., Nijman, S.M., Dirac, A.M. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003).
Boone, D.L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Lefkowitz, R.J. & Whalen, E.J. β-Arrestins: traffic cops of cell signaling. Curr. Opin. Cell Biol. 16, 162–168 (2004).
Wilbanks, A.M. et al. β-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science 306, 2264–2267 (2004).
Chen, W. et al. Activity-dependent internalization of smoothened mediated by β-arrestin 2 and GRK2. Science 306, 2257–2260 (2004).
Perry, S.J. & Lefkowitz, R.J. Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol. 12, 130–138 (2002).
Chen, W. β-Arrestin 2 mediates endocytosis of type III TGF-β receptor and down-regulation of its signaling. Science 301, 1394–1397 (2003).
Chen, W. et al. Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301, 1391–1394 (2003).
Luttrell, L.M., Daaka, Y. & Lefkowitz, R.J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 11, 177–183 (1999).
McDonald, P.H. et al. β-Arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574–1577 (2000).
Shenoy, S.K., McDonald, P.H., Kohout, T.A. & Lefkowitz, R.J. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science 294, 1307–1313 (2001).
Wang, P. et al. β-Arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J. Biol. Chem. 278, 6363–6370 (2003).
Gao, H. et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol. Cell 14, 303–317 (2004).
Sun, L., Deng, L., Ea, C.K., Xia, Z.P. & Chen, Z.J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Kobayashi, T., Walsh, M.C. & Choi, Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 6, 1333–1338 (2004).
Ye, H. et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418, 443–447 (2002).
Li, X., Yang, Y. & Ashwell, J.D. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 416, 345–347 (2002).
Sato, S. et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171, 4304–4310 (2003).
Jiang, Z. et al. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NF-κB and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6–TAK1-TAB2-PKR. J. Biol. Chem. 278, 16713–16719 (2003).
Jiang, Z., Mak, T.W., Sen, G. & Li, X. Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc. Natl. Acad. Sci. USA 101, 3533–3538 (2004).
Xu, L.G. et al. VISA Is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 (2005).
Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18, 6853–6866 (1999).
Janeway, C.A., Jr. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Akira, S. Toll-like receptor signaling. J. Biol. Chem. 278, 38105–38108 (2003).
Barton, G.M. & Medzhitov, R. Toll-like receptor signaling pathways. Science 300, 1524–1525 (2003).
Liew, F.Y., Xu, D., Brint, E.K. & O'Neill, L.A. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Bohn, L.M., Gainetdinov, R.R., Lin, F.T., Lefkowitz, R.J. & Caron, M.G. μ-opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408, 720–723 (2000).
Bohn, L.M. et al. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 286, 2495–2498 (1999).
Beaulieu, J.M. et al. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273 (2005).
Witherow, D.S., Garrison, T.R., Miller, W.E. & Lefkowitz, R.J. β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc. Natl. Acad. Sci. USA 101, 8603–8607 (2004).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Acknowledgements
We thank R.J. Lefkowitz for anti-β-arrestin, MEF cell lines and β-arrestin 2–deficient mice; H. Shu for TRAF1–TRAF6 and TLR3 expression constructs; D. Bohmann for the ubiquitin plasmid; and B. Su for reading the manuscript. Supported by the Ministry of Science and Technology, the National Natural Science Foundation of China and the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic representation of wild-type and deletion mutants of TRAF6 and β-arrestins. (PDF 376 kb)
Supplementary Fig. 2
The TRAF-N domain was required for TRAF6 autoubiquitination and oligomerization. (PDF 296 kb)
Supplementary Fig. 3
IKK activity was augmented in bone marrow-derived macrophages (BMDMs) from Arrb2−/− mice. (PDF 127 kb)
Supplementary Fig. 4
Endogenous TRAF6 autoubiquitination was detected in Arrb−/− MEFs and HeLa cells. (PDF 312 kb)
Supplementary Fig. 5
β-Arrestins regulated cytokine production in HeLa cells. (PDF 236 kb)
Supplementary Fig. 6
Expression of β-arrestins was unaffected in macrophages after LPS stimulation. (PDF 114 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Tang, Y., Teng, L. et al. Association of β-arrestin and TRAF6 negatively regulates Toll-like receptor–interleukin 1 receptor signaling. Nat Immunol 7, 139–147 (2006). https://doi.org/10.1038/ni1294
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1294
This article is cited by
-
HTR2A agonists play a therapeutic role by restricting ILC2 activation in papain-induced lung inflammation
Cellular & Molecular Immunology (2023)
-
New insights into the genetic predisposition of brucellosis and its effect on the gut and vaginal microbiota in goats
Scientific Reports (2023)
-
β‐arrestin 2 negatively regulates lung cancer progression by inhibiting the TRAF6 signaling axis for NF-κB activation and autophagy induced by TLR3 and TLR4
Cell Death & Disease (2023)
-
Targets and regulation of microRNA-652-3p in homoeostasis and disease
Journal of Molecular Medicine (2021)
-
β-arrestin 2 as an activator of cGAS-STING signaling and target of viral immune evasion
Nature Communications (2020)