Abstract
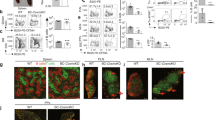
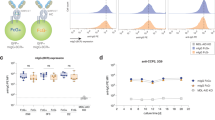
CD22 is a negative regulator of B cell signaling, an activity modulated by its interaction with glycan ligands containing α2-6-linked sialic acids. B cells deficient in the enzyme (ST6Gal I) that forms the CD22 ligand show suppressed BCR signaling. Here we report that mice deficient in both CD22 and its ligand (Cd22−/−St6gal1−/− mice) showed restored B cell receptor (BCR) signaling, suggesting that the suppressed signaling of St6gal1−/− cells is mediated through CD22. Coincident with suppressed BCR signaling, B cells lacking ST6Gal I showed a net redistribution of the BCR to clathrin-rich microdomains containing most of the CD22, resulting in a twofold increase in the localization of CD22 together with the BCR. These studies suggest an important function for the CD22-ligand interaction in regulating BCR signaling and microdomain localization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tsubata, T. & Wienands, J. B cell signaling. Introduction. Int. Rev. Immunol. 20, 675–678 (2001).
Tedder, T.F., Poe, J.C. & Haas, K.M. CD22: A Multifunctional Receptor That Regulates B Lymphocyte Survival and Signal Transduction. Adv. Immunol. 88, 1–50 (2005).
Tedder, T.F., Tuscano, J., Sato, S. & Kehrl, J.H. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu. Rev. Immunol. 15, 481–504 (1997).
Cyster, J.G. & Goodnow, C.C. Tuning antigen receptor signaling by CD22: integrating cues from antigens and the microenvironment. Immunity 6, 509–517 (1997).
Nitschke, L., Carsetti, R., Ocker, B., Kohler, G. & Lamers, M.C. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 7, 133–143 (1997).
O'Keefe, T.L., Williams, G.T., Batista, F.D. & Neuberger, M.S. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J. Exp. Med. 189, 1307–1313 (1999).
O'Keefe, T.L., Williams, G.T., Davies, S.L. & Neuberger, M.S. Hyperresponsive B cells in CD22-deficient mice. Science 274, 798–801 (1996).
Otipoby, K.L. et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature 384, 634–637 (1996).
Sato, S. et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity 5, 551–562 (1996).
Blasioli, J., Paust, S. & Thomas, M.L. Definition of the sites of interaction between the protein tyrosine phosphatase SHP-1 and CD22. J. Biol. Chem. 274, 2303–2307 (1999).
Doody, G.M. et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269, 242–244 (1995).
Cornall, R.J. et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity 8, 497–508 (1998).
Smith, K.G., Tarlinton, D.M., Doody, G.M., Hibbs, M.L. & Fearon, D.T. Inhibition of the B cell by CD22: a requirement for Lyn. J. Exp. Med. 187, 807–811 (1998).
Zhang, M. & Varki, A. Cell surface sialic acids do not affect primary CD22 interactions with CD45 and surface IgM nor the rate of constitutive CD22 endocytosis. Glycobiology 14, 939–949 (2004).
Phee, H., Rodgers, W. & Coggeshall, K.M. Visualization of negative signaling in B cells by quantitative confocal microscopy. Mol. Cell. Biol. 21, 8615–8625 (2001).
Peaker, C.J. & Neuberger, M.S. Association of CD22 with the B cell antigen receptor. Eur. J. Immunol. 23, 1358–1363 (1993).
Law, C.L., Sidorenko, S.P. & Clark, E.A. Regulation of lymphocyte activation by the cell-surface molecule CD22. Immunol. Today 15, 442–449 (1994).
Leprince, C., Draves, K.E., Geahlen, R.L., Ledbetter, J.A. & Clark, E.A. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc. Natl. Acad. Sci. USA 90, 3236–3240 (1993).
Crocker, P.R. Siglecs in innate immunity. Curr. Opin. Pharmacol. 5, 431–437 (2005).
Varki, A. & Angata, T. Siglecs - the Major Sub-family of I-type Lectins. Glycobiology (2005).
Crocker, P.R. & Varki, A. Siglecs in the immune system. Immunology 103, 137–145 (2001).
Kelm, S. et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4, 965–972 (1994).
Powell, L.D., Jain, R.K., Matta, K.L., Sabesan, S. & Varki, A. Characterization of sialyloligosaccharide binding by recombinant soluble and native cell-associated CD22. Evidence for a minimal structural recognition motif and the potential importance of multisite binding. J. Biol. Chem. 270, 7523–7532 (1995).
Blixt, O., Collins, B.E., van den Nieuwenhof, I.M., Crocker, P.R. & Paulson, J.C. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278, 31007–31019 (2003).
Lo, N.W. & Lau, J.T. Transcription of the β-galactoside α2,6-sialyltransferase gene (SIAT1) in B-lymphocytes: cell type-specific expression correlates with presence of the divergent 5′-untranslated sequence. Glycobiology 9, 907–914 (1999).
Kitagawa, H. & Paulson, J.C. Differential expression of five sialyltransferase genes in human tissues. J. Biol. Chem. 269, 17872–17878 (1994).
Hennet, T., Chui, D., Paulson, J.C. & Marth, J.D. Immune regulation by the ST6Gal sialyltransferase. Proc. Natl. Acad. Sci. USA 95, 4504–4509 (1998).
Sgroi, D., Varki, A., Braesch-Andersen, S. & Stamenkovic, I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J. Biol. Chem. 268, 7011–7018 (1993).
Law, C.L., Aruffo, A., Chandran, K.A., Doty, R.T. & Clark, E.A. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. J. Immunol. 155, 3368–3376 (1995).
Han, S., Collins, B.E., Bengston, P. & Paulson, J.C. Homo-multimeric complexes of CD22 in B cells revealed by protein-glycan crosslinking. Nat. Chem. Biol. 1, 93–97 (2005).
Collins, B.E. et al. Constitutively unmasked CD22 on B cells of ST6Gal I knockout mice: novel sialoside probe for murine CD22. Glycobiology 12, 563–571 (2002).
Razi, N. & Varki, A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl. Acad. Sci. USA 95, 7469–7474 (1998).
Danzer, C.P., Collins, B.E., Blixt, O., Paulson, J.C. & Nitschke, L. Transitional and marginal zone B cells have a high proportion of unmasked CD22: implications for BCR signaling. Int. Immunol. 15, 1137–1147 (2003).
Jin, L., McLean, P.A., Neel, B.G. & Wortis, H.H. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J. Exp. Med. 195, 1199–1205 (2002).
Kelm, S., Gerlach, J., Brossmer, R., Danzer, C.P. & Nitschke, L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 195, 1207–1213 (2002).
Poe, J.C. et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat. Immunol. 5, 1078–1087 (2004).
Crocker, P.R. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 (2002).
Nitschke, L. & Tsubata, T. Molecular interactions regulate BCR signal inhibition by CD22 and CD72. Trends Immunol. 25, 543–550 (2004).
Smith, K.G. & Fearon, D.T. Receptor modulators of B-cell receptor signalling–CD19/CD22. Curr. Top. Microbiol. Immunol. 245, 195–212 (2000).
Lajaunias, F. et al. Differential control of CD22 ligand expression on B and T lymphocytes, and enhanced expression in murine systemic lupus. Arthritis Rheum. 48, 1612–1621 (2003).
Samardzic, T. et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 32, 561–567 (2002).
Nadler, M.J., McLean, P.A., Neel, B.G. & Wortis, H.H. B cell antigen receptor-evoked calcium influx is enhanced in CD22-deficient B cell lines. J. Immunol. 159, 4233–4243 (1997).
Lenschow, D.J. et al. Differential up-regulation of the B7–1 and B7–2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 153, 1990–1997 (1994).
Dykstra, M., Cherukuri, A. & Pierce, S.K. Rafts and synapses in the spatial organization of immune cell signaling receptors. J. Leukoc. Biol. 70, 699–707 (2001).
Vallejo, J. & Hardin, C.D. Expression of caveolin-1 in lymphocytes induces caveolae formation and recruitment of phosphofructokinase to the plasma membrane. FASEB J. 19, 586–587 (2005).
Hanasaki, K., Powell, L.D. & Varki, A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22. Selective recognition of immunoglobulin M and haptoglobin. J. Biol. Chem. 270, 7543–7550 (1995).
Poe, J.C. et al. Severely impaired B lymphocyte proliferation, survival, and induction of the c-Myc:Cullin 1 ubiquitin ligase pathway resulting from CD22 deficiency on the C57BL/6 genetic background. J. Immunol. 172, 2100–2110 (2004).
Stoddart, A. et al. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462 (2002).
Stoddart, A., Jackson, A.P. & Brodsky, F.M. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol. Biol. Cell 16, 2339–2348 (2005).
Pierce, S.K. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2, 96–105 (2002).
Cherukuri, A., Cheng, P.C. & Pierce, S.K. The role of the CD19/CD21 complex in B cell processing and presentation of complement-tagged antigens. J. Immunol. 167, 163–172 (2001).
Cherukuri, A. et al. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J. Immunol. 172, 370–380 (2004).
John, B. et al. The B cell coreceptor CD22 associates with AP50, a clathrin-coated pit adapter protein, via tyrosine-dependent interaction. J. Immunol. 170, 3534–3543 (2003).
Pflugh, D.L., Maher, S.E. & Bothwell, A.L. Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J. Immunol. 169, 5130–5136 (2002).
Jascur, T. et al. Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding TPR protein. Mol. Cell 17, 237–249 (2005).
Acknowledgements
We thank J. Marth and L. Nitschke for ST6Gal I–deficient and CD22-deficient mice, respectively; H. Li and M. Iufer for technical assistance and A. Tran-Crie for assistance in manuscript preparation. Supported by the National Institutes of Health (AI050143 to J.C.P. and GM25042 to B.E.C.) and the Wenner-Gren Foundation (P.B.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Restored phosphorylation of cytoplasmic proteins in B cells from double-knockout mice. (PDF 521 kb)
Supplementary Fig. 2
Similar CD22 and IgM co-clustering following BCR crosslinking on B cells from WT or ST6Gal I null mice. (PDF 89 kb)
Rights and permissions
About this article
Cite this article
Collins, B., Smith, B., Bengtson, P. et al. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol 7, 199–206 (2006). https://doi.org/10.1038/ni1283
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1283
This article is cited by
-
The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans
Nature Reviews Drug Discovery (2021)
-
Sialylation of immunoglobulin E is a determinant of allergic pathogenicity
Nature (2020)
-
Cytoskeletal control of B cell responses to antigens
Nature Reviews Immunology (2017)
-
Sialylation of N-glycans: mechanism, cellular compartmentalization and function
Histochemistry and Cell Biology (2017)
-
CD22 and CD72 are inhibitory receptors dominantly expressed in B lymphocytes and regulate systemic autoimmune diseases
Zeitschrift für Rheumatologie (2017)