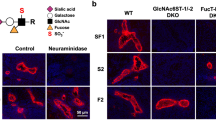
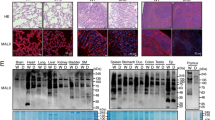
Abstract
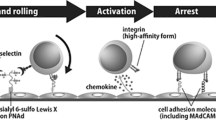
The interaction of L-selectin on lymphocytes with sulfated ligands on high endothelial venules leads to rolling and is critical for recruitment of lymphocytes into peripheral lymph nodes. Peripheral node addressin represents a class of L-selectin ligands recognized by the function-blocking monoclonal antibody MECA-79. Its epitope overlaps with sialyl 6-sulfo Lewis X, an L-selectin recognition determinant. Here, mice lacking two N-acetylglucosamine-6-O-sulfotransferases (GlcNAc6ST-1 and GlcNAc6ST-2) demonstrated elimination of both peripheral node addressin and sialyl 6-sulfo Lewis X in high endothelial venules, considerably reduced lymphocyte homing to peripheral lymph nodes and reduced sticking of lymphocytes along high endothelial venules. Our results establish an essential function for the sulfotransferases in L-selectin ligand synthesis and may have relevance for therapy of inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Butcher, E.C. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell 67, 1033–1036 (1991).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Rosen, S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Streeter, P.R., Rouse, B.T. & Butcher, E.C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
van Zante, A. et al. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. J. Exp. Med. 198, 1289–1300 (2003).
von Andrian, U.H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996).
Renkonen, J., Tynninen, O., Hayry, P., Paavonen, T. & Renkonen, R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am. J. Pathol. 161, 543–550 (2002).
Lowe, J.B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 186, 19–36 (2002).
Rosen, S.D., Singer, M.S., Yednock, T.A. & Stoolman, L.M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science 228, 1005–1007 (1985).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Imai, Y., Lasky, L.A. & Rosen, S.D. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature 361, 555–557 (1993).
Hemmerich, S., Bertozzi, C.R., Leffler, H. & Rosen, S.D. Identification of the sulfated monosaccharides of GlyCAM-1, an endothelial-derived ligand for L-selectin. Biochemistry 33, 4820–4829 (1994).
Yeh, J.C. et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3-N-acetylglucosaminyltransferase. Cell 105, 957–969 (2001).
Satomaa, T. et al. O-glycans on human high endothelial CD34 putatively participating in L-selectin recognition. Blood 99, 2609–2611 (2002).
Mitsuoka, C. et al. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J. Biol. Chem. 273, 11225–11233 (1998).
Hemmerich, S. et al. Chromosomal localization and genomic organization for the galactose/ N-acetylgalactosamine/N-acetylglucosamine 6-O-sulfotransferase gene family. Glycobiology 11, 75–87 (2001).
Grunwell, J.R. & Bertozzi, C.R. Carbohydrate sulfotransferases of the GalNAc/Gal/GlcNAc6ST family. in Biochemistry 41, 13117–13126 (2002).
Uchimura, K. et al. Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J. Biol. Chem. 273, 22577–22583 (1998).
Uchimura, K. et al. Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis X: molecular cloning, chromosomal mapping, and expression in various organs and tumor cells. J. Biochem. 124, 670–678 (1998).
Li, X. & Tedder, T.F. CHST1 and CHST2 sulfotransferases expressed by human vascular endothelial cells: cDNA cloning, expression, and chromosomal localization. Genomics 55, 345–347 (1999).
Bistrup, A. et al. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J. Cell Biol. 145, 899–910 (1999).
Hiraoka, N. et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis(x), an L-selectin ligand displayed by CD34. Immunity 11, 79–89 (1999).
Uchimura, K. et al. Specificities of N-acetylglucosamine-6-O-sulfotransferases in relation to L-selectin ligand synthesis and tumor-associated enzyme expression. J. Biol. Chem. 277, 3979–3984 (2002).
Rosen, S.D., Tsay, D., Singer, M.S., Hemmerich, S. & Abraham, W.M. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am. J. Pathol. 166, 935–944 (2005).
Kimura, N. et al. Reconstitution of functional L-selectin ligands on a cultured human endothelial cell line by cotransfection of α1 → 3 fucosyltransferase VII and newly cloned GlcNAcβ:6-sulfotransferase cDNA. Proc. Natl. Acad. Sci. USA 96, 4530–4535 (1999).
de Graffenried, C.L. & Bertozzi, C.R. Golgi localization of carbohydrate sulfotransferases is a determinant of L-selectin ligand biosynthesis. J. Biol. Chem. 278, 40282–40295 (2003).
Hemmerich, S. et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 15, 237–247 (2001).
Uchimura, K. et al. N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing. J. Biol. Chem. 279, 35001–35008 (2004).
Duijvestijn, A.M., Schreiber, A.B. & Butcher, E.C. Interferon-γ regulates an antigen specific for endothelial cells involved in lymphocyte traffic. Proc. Natl. Acad. Sci. USA 83, 9114–9118 (1986).
Bistrup, A. et al. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope. Am. J. Pathol. 164, 1635–1644 (2004).
Hemmerich, S., Butcher, E.C. & Rosen, S.D. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. J. Exp. Med. 180, 2219–2226 (1994).
Gallatin, W., Weissman, I. & Butcher, E. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304, 30–34 (1983).
Bargatze, R.F., Jutila, M.A. & Butcher, E.C. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3, 99–108 (1995).
Kunkel, E.J. et al. The roles of L-selectin, β7 integrins, and P-selectin in leukocyte rolling and adhesion in high endothelial venules of Peyer's patches. in J. Immunol. 161, 2449–2456 (1998).
Streeter, P.R., Berg, E.L., Rouse, B.T., Bargatze, R.F. & Butcher, E.C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331, 41–46 (1988).
Tangemann, K., Bistrup, A., Hemmerich, S. & Rosen, S.D. Sulfation of a high endothelial venule-expressed ligand for L-selectin. Effects on tethering and rolling of lymphocytes. J. Exp. Med. 190, 935–942 (1999).
Mebius, R.E. & Watson, S.R. L- and E-selectin can recognize the same naturally occurring ligands on high endothelial venules. J. Immunol. 151, 3252–3260 (1993).
Wang, L., Fuster, M., Sriramarao, P. & Esko, J.D. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 6, 902–910 (2005).
Nelson, R.M. et al. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 82, 3253–3258 (1993).
Gauguet, J.M., Rosen, S.D., Marth, J.D. & von Andrian, U.H. Core 2 branching β1,6-N-acetylglucosaminyltransferase and high endothelial cell N-acetylglucosamine-6-sulfotransferase exert differential control over B- and T-lymphocyte homing to peripheral lymph nodes. Blood 104, 4104–4112 (2004).
Stein, J.V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Rosen, S.D. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am. J. Pathol. 155, 1013–1020 (1999).
Kawashima, H. et al. N-acetylglucosamine-6-O-sulfotransferase-1 and -2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. advance online publication 9 October 2005 (1038/ni 1259).
Lee, J.K., Bistrup, A., van Zante, A. & Rosen, S.D. Activities and expression pattern of the carbohydrate sulfotransferase GlcNAc6ST-3 (I-GlcNAc6ST): functional implications. Glycobiology 13, 245–254 (2003).
Hiraoka, N. et al. Core 2 branching β1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J. Biol. Chem. 279, 3058–3067 (2004).
M'Rini, C. et al. A novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by α(1,3)-fucosyltransferase-IV. J. Exp. Med. 198, 1301–1312 (2003).
Hemmerich, S. & Rosen, S.D. 6'-sulfated sialyl Lewis x is a major capping group of GlyCAM-1. Biochemistry 33, 4830–4835 (1994).
Hemmerich, S., Leffler, H. & Rosen, S.D. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J. Biol. Chem. 270, 12035–12047 (1995).
Torii, T., Fukuta, M. & Habuchi, O. Sulfation of sialyl N-acetyllactosamine oligosaccharides and fetuin oligosaccharides by keratan sulfate Gal-6-sulfotransferase. Glycobiology 10, 203–211 (2000).
Galkina, E. et al. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J. Exp. Med. 198, 1323–1335 (2003).
Izawa, M. et al. Expression of sialyl 6-sulfo Lewis X is inversely correlated with conventional sialyl Lewis X expression in human colorectal cancer. Cancer Res. 60, 1410–1416 (2000).
Manjunath, N. et al. A transgenic mouse model to analyze CD8+ effector T cell differentiation in vivo. Proc. Natl. Acad. Sci. USA 96, 13932–13937 (1999).
von Andrian, U.H. & M'Rini, C. In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes. Commun. 6, 85–96 (1998).
Pries, A.R. A versatile video image analysis system for microcirculatory research. Int. J. Microcirc. Clin. Exp. 7, 327–345 (1988).
Acknowledgements
We thank A. Bistrup for discussions; Y.-Q. Wang for assistance with maintenance of the mouse colony, and M. Izawa for technical support. Supported by the National Institutes of Health (R01-GM57411 and R37-GM23547 to S.D.R. and HL54936, AI061663 and HL56949 to U.H.v.A.) the Japan Society for the Promotion of Science (K.U.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Uchimura, K., Gauguet, JM., Singer, M. et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol 6, 1105–1113 (2005). https://doi.org/10.1038/ni1258
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1258
This article is cited by
-
Immunomodulation by endothelial cells — partnering up with the immune system?
Nature Reviews Immunology (2022)
-
A defined glycosylation regulatory network modulates total glycome dynamics during pluripotency state transition
Scientific Reports (2021)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
Preferential expression of sialyl 6′-sulfo N-acetyllactosamine-capped O-glycans on high endothelial venules in human peripheral lymph nodes
Laboratory Investigation (2019)
-
GlcNAc6ST3 is a keratan sulfate sulfotransferase for the protein-tyrosine phosphatase PTPRZ in the adult brain
Scientific Reports (2019)