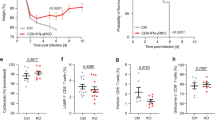
Abstract
Several studies have indicated that CD8+ T cells require CD4+ T cell help for memory formation. Evidence suggests that such help can be antigen independent, challenging whether the 'licensing' of dendritic cells (DCs) by CD4+ T cells is ever required for cytotoxic T lymphocyte (CTL) responses. We show here that help is essential for the generation of CTL immunity to herpes simplex virus 1 and that CD4+ T cells mediate help in a cognate, antigen-specific way. We provide direct in vivo evidence for DC licensing by helper T cells and show that licensing is rapid and essential for the formation of effector and memory CTLs. In situations in which DCs are poorly licensed by pathogen-derived signals, our findings suggest that CTL immunity may be heavily dependent on cognate DC licensing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bevan, M.J. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4, 595–602 (2004).
Bourgeois, C., Rocha, B. & Tanchot, C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297, 2060–2063 (2002).
Janssen, E.M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Sun, J.C. & Bevan, M.J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Shedlock, D.J. & Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300, 337–339 (2003).
Sun, J.C., Williams, M.A. & Bevan, M.J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5, 927–933 (2004).
Jennings, S.R., Bonneau, R.H., Smith, P.M., Wolcott, R.M. & Chervenak, R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell. Immunol. 133, 234–252 (1991).
Bennett, S.R. et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393, 478–480 (1998).
Bennett, S.R., Carbone, F.R., Karamalis, F., Miller, J.F. & Heath, W.R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186, 65–70 (1997).
Marzo, A.L. et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 173, 969–975 (2004).
Husmann, L.A. & Bevan, M.J. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann. NY Acad. Sci. 532, 158–169 (1988).
Guerder, S. & Matzinger, P. A fail-safe mechanism for maintaining self-tolerance. J. Exp. Med. 176, 553–564 (1992).
Krieger, N.R., Yin, D.P. & Garrison Fathman, C. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 184, 2013–2018 (1996).
Schoenberger, S.P., Toes, R.E., van der Voort, E.I., Offringa, R. & Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 393, 480–483 (1998).
Cassell, D. & Forman, J. Linked recognition of helper and cytotoxic antigenic determinants for the generation of cytotoxic T lymphocytes. Ann. NY Acad. Sci. 532, 51–60 (1988).
Mitchison, N.A. & O'Malley, C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur. J. Immunol. 17, 1579–1583 (1987).
Ridge, J.P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 (1998).
Schuurhuis, D.H. et al. Immature dendritic cells acquire CD8+ cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J. Exp. Med. 192, 145–150 (2000).
Belz, G.T., Wodarz, D., Diaz, G., Nowak, M.A. & Doherty, P.C. Compromised influenza virus-specific CD8+-T-cell memory in CD4+-T-cell-deficient mice. J. Virol. 76, 12388–12393 (2002).
Sun, J.C. & Bevan, M.J. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J. Immunol. 172, 3385–3389 (2004).
Le Bon, A. et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4, 1009–1015 (2003).
Wang, J.C. & Livingstone, A.M. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J. Immunol. 171, 6339–6343 (2003).
Behrens, G.M. et al. Helper requirements for generation of effector CTL to islet beta cell antigens. J. Immunol. 172, 5420–5426 (2004).
Allan, R.S. et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 (2003).
Heath, W.R. et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199, 9–26 (2004).
Smith, C.M. et al. Cutting edge: Conventional CD8α+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J. Immunol. 170, 4437–4440 (2003).
Belz, G.T. et al. Cutting edge: Conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172, 1996–2000 (2004).
Kaech, S.M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003).
Lauvau, G. et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294, 1735–1739 (2001).
Ahmed, R., Butler, L.D. & Bhatti, L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J. Virol. 62, 2102–2106 (1988).
Buller, R.M., Holmes, K.L., Hugin, A., Frederickson, T.N. & Morse, H.C.D. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328, 77–79 (1987).
Liu, Y. & Mullbacher, A. The generation and activation of memory class I MHC restricted cytotoxic T cell responses to influenza A virus in vivo do not require CD4+ T cells. Immunol. Cell Biol. 67, 413–420 (1989).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Weiss, E.H. et al. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature 301, 671–674 (1983).
Gosgrove, D. et al. Mice lacking MHC class II molecules. Cell 66, 1051–1066 (1991).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Mueller, S.N., Heath, W.R., Carbone, F.R. & Jones, C.M. The characterisation of two transgenic mice specific for herpes simplex virus. Immunol. Cell Biol. 80, 156–163 (2002).
Mueller, S.N., Jones, C.M., Smith, C.M., Heath, W.R. & Carbone, F.R. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195, 651–656 (2002).
Jones, C.M. et al. Herpes simplex virus type 1-specific cytotoxic T-lymphocyte arming occurs within lymph nodes draining the site of cutaneous infection. J. Virol. 74, 2414–2419 (2000).
Sherman, L.A. & Randolph, C.P. Monoclonal anti-H-2Kb antibodies detect serological differences between H-2Kb mutants. Immunogenetics 12, 183–186 (1981).
Acknowledgements
We thank K. Shortman and D. Huang for providing antibodies; J.L. Tan, J. Langley, L.M. Kleinert, M. Camilleri and the staff of the Walter and Eliza Hall Institute flow cytometry facility for technical assistance. Supported by the National Health and Medical Research Institute (Australia), Howard Hughes Medical Institute and Wellcome Trust Medical Research Fund (UK).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
tetramer-positive CD8+ T cell responses to subcutaneous HSV infection requires CD4+ T cell help. (PDF 21 kb)
Supplementary Fig. 2
Efficient gB-specific cytotoxicity to HSV after subcutaneous infection requires CD4+ T cell help. (PDF 103 kb)
Supplementary Fig. 3
CD8 DCs are licensed by 26 h after infection as measured by the ability to stimulate proliferation of OT-I cells. (PDF 115 kb)
Supplementary Fig. 4
CD8 DCs are licensed by 26 h after infection as measured by upregulation of IL-7 receptor on gBT-I cells. (PDF 81 kb)
Rights and permissions
About this article
Cite this article
Smith, C., Wilson, N., Waithman, J. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat Immunol 5, 1143–1148 (2004). https://doi.org/10.1038/ni1129
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1129
This article is cited by
-
Immunity to Cryptosporidium: insights into principles of enteric responses to infection
Nature Reviews Immunology (2024)
-
A hepatic network of dendritic cells mediates CD4 T cell help outside lymphoid organs
Nature Communications (2024)
-
Immune synapse formation promotes lipid peroxidation and MHC-I upregulation in licensed dendritic cells for efficient priming of CD8+ T cells
Nature Communications (2023)
-
Dendritic cells in cancer immunology
Cellular & Molecular Immunology (2022)
-
Tissue resident memory T cells inhabit the deep human conjunctiva
Scientific Reports (2022)