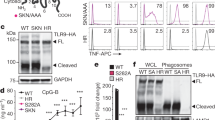
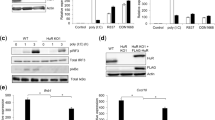
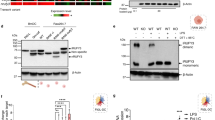
Abstract
Microbial DNA sequences containing unmethylated CpG dinucleotides activate Toll-like receptor 9 (TLR9). We have found that TLR9 is localized to the endoplasmic reticulum (ER) of dendritic cells (DCs) and macrophages. Because there is no precedent for immune receptor signaling in the ER, we investigated how TLR9 is activated. We show that CpG DNA binds directly to TLR9 in ligand-binding studies. CpG DNA moves into early endosomes and is subsequently transported to a tubular lysosomal compartment. Concurrent with the movement of CpG DNA in cells, TLR9 redistributes from the ER to CpG DNA–containing structures, which also accumulate MyD88. Our data indicate a previously unknown mechanism of cellular activation involving the recruitment of TLR9 from the ER to sites of CpG DNA uptake, where signal transduction is initiated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tokunaga, T. et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J. Natl. Cancer Inst. 72, 955–962 (1984).
Yamamoto, S., Kuramoto, E., Shimada, S. & Tokunaga, T. In vitro augmentation of natural killer cell activity and production of interferon-α/β and -γ with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn. J. Cancer Res. 79, 866–873 (1988).
Krieg, A.M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).
Agrawal, S. & Kandimalla, E.R. Medicinal chemistry and therapeutic potential of CpG DNA. Trends Mol. Med. 8, 114–121 (2002).
Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20, 709–760 (2002).
Gordon, S. Pattern recognition receptors: doubling up for the innate immune response. Cell 111, 927–930 (2002).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Bauer, S. et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98, 9237–9242 (2001).
Tauszig, S., Jouanguy, E., Hoffmann, J.A. & Imler, J.L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA 97, 10520–10525 (2000).
Weber, A.N. et al. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat. Immunol. 4, 794–800 (2003).
Manzel, L. & Macfarlane, D.E. Lack of immune stimulation by immobilized CpG-oligodeoxynucleotide. Antisense Nucleic Acid Drug. Dev. 9, 459–464 (1999).
Yamamoto, T., Yamamoto, S., Kataoka, T. & Tokunaga, T. Lipofection of synthetic oligodeoxyribonucleotide having a palindromic sequence of AACGTT to murine splenocytes enhances interferon production and natural killer activity. Microbiol. Immunol. 38, 831–836 (1994).
Gursel, I., Gursel, M., Ishii, K.J. & Klinman, D.M. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J. Immunol. 167, 3324–3328 (2001).
Hacker, H. et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 17, 6230–6240 (1998).
Yi, A.K. et al. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J. Immunol. 160, 4755–4761 (1998).
Ahmad-Nejad, P. et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32, 1958–1968 (2002).
Takeshita, F. et al. Cutting edge: Role of Toll-like receptor 9 in CpG DNA–induced activation of human cells. J. Immunol. 167, 3555–3558 (2001).
Latz, E. et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4–MD-2–CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277, 47834–47843 (2002).
Hornung, V. et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537 (2002).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Stenmark, H., Aasland, R. & Driscoll, P.C. The phosphatidylinositol 3-phosphate-binding FYVE finger. FEBS Lett. 513, 77–84 (2002).
Helenius, A. & Aebi, M. Intracellular functions of N-linked glycans. Science 291, 2364–2369 (2001).
Desjardins, M. ER-mediated phagocytosis: a new membrane for new functions. Nat. Rev. Immunol. 3, 280–291 (2003).
Gagnon, E. et al. Endoplasmic reticulum–mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131 (2002).
Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9–mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003).
Flo, T.H. et al. Involvement of Toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J. Biol. Chem. 277, 35489–35495 (2002).
Underhill, D.M. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999).
Lee, J. et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 100, 6646–6651 (2003).
Mellman, I. Endocytosis and molecular sorting. Annu. Rev. Cell. Dev. Biol. 12, 575–625 (1996).
Garin, J. et al. The phagosome proteome: insight into phagosome functions. J. Cell. Biol. 152, 165–180 (2001).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003).
Okazaki, Y., Ohno, H., Takase, K., Ochiai, T. & Saito, T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J. Biol. Chem. 275, 35751–35758 (2000).
Johnson, S., Michalak, M., Opas, M. & Eggleton, P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell. Biol. 11, 122–129 (2001).
Leadbetter, E.A. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Chow, A., Toomre, D., Garrett, W. & Mellman, I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).
Acknowledgements
We thank K. Halmen, L. Ryan, R. Vik and K. Egeberg for technical support; S. Akira (Osaka University, Osaka, Japan) for the full-length TLR9 cDNA and the MyD88-deficient mice; S. Ishizaka (Eisai Research Institute, Andover, Massachusetts, USA) for the HEK–TLR9–NF-κB–luc reporter cell line; B. Seed (Harvard University and Massachusetts General Hospital, Boston, Massachusetts, USA) for the retroviral vector peak12mmp; N. Aliverdi (eBiosciences, San Diego, California, USA) for TLR9 mAbs; A. Sundan (Norwegian Institute of Science and Technology, Trondheim, Norway) for antibody labeling; and H. Stenmark (Norwegian Radium Hospital, Oslo, Norway) for the FYVE-GFP construct. This work was supported by grants from the National Institute of Health (D.T.G), the Commission of the European Communities (T.E. and D.T.G.), the Norwegian Research Council and the Norwegian Cancer Society (T.E.), and by the German Academic Exchange Program (E.L).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Latz, E., Schoenemeyer, A., Visintin, A. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5, 190–198 (2004). https://doi.org/10.1038/ni1028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1028
This article is cited by
-
Mitochondria and cell death-associated inflammation
Cell Death & Differentiation (2023)
-
Extra-viral DNA in adeno-associated viral vector preparations induces TLR9-dependent innate immune responses in human plasmacytoid dendritic cells
Scientific Reports (2023)
-
Activation of immune signals during organ transplantation
Signal Transduction and Targeted Therapy (2023)
-
Nanoparticulate cell-free DNA scavenger for treating inflammatory bone loss in periodontitis
Nature Communications (2022)
-
Tuning the Stability of the Polyplex Nanovesicles of Oligonucleotides via a Zinc (II)-Coordinative Strategy
Chinese Journal of Polymer Science (2022)