Abstract
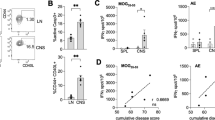
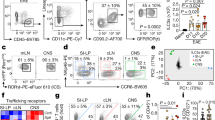
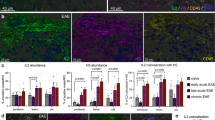
The inducible costimulatory molecule (ICOS) is expressed on activated T cells and participates in a variety of important immunoregulatory functions. After the induction of experimental allergic encephalomyelitis in SJL mice with proteolipid protein (PLP), brain ICOS mRNA and protein were up-regulated on infiltrating CD3+ T cells before disease onset. ICOS blockade during the efferent immune response (9–20 days after immunization) abrogated disease, but blockade during antigen priming (1–10 days after immunization) exacerbated disease. Upon culture with PLP and compared with immunized controls, splenocytes produced either decreased interferon-γ (IFN-γ, in efferent blockade) or excessive IFN-γ (in priming blockade). PLP-specific immunoglobulin G1 was decreased in animals treated with anti-ICOS during antigen priming, but not in other groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Perrin, P. J. et al. Blockade of CD28 during in vitro activation of encephalitogenic T cells or after disease onset ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 163, 1704–1710 (1999).
Tada, Y. et al. CD28-deficient mice are highly resistant to collagen-induced arthritis. J. Immunol. 162, 203–208 (1999).
Mathur, M. et al. CD28 interactions with either CD80 or CD86 are sufficient to induce allergic airway inflammation in mice. Am. J. Respir. Cell. Mol. Biol. 21, 498–509 (1999).
Kopf, M. et al. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192, 53–61 (2000).
McAdam, A. J. et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4(+) T cells. J. Immunol. 165, 5035–5040 (2000).
Yoshinaga, S. K. et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402, 827–832 (1999).
Mages, H. W. et al. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur. J. Immunol. 30, 1040–1047 (2000).
Coyle, A. J. et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 13, 95–105 (2000).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
McAdam, A. J. et al. ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 (2001).
Dong, C. et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409, 97–101 (2001).
Tafuri, A. et al. ICOS is essential for effective T-helper-cell responses. Nature 409, 105–109 (2001).
Wekerle, H. Immunopathogenesis of multiple sclerosis. Acta. Neurol. Napoli 13, 197–204 (1991).
Rottman, J. B. et al. Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur. J. Immunol. 30, 2372–2377 (2000).
Karpus, W. J. et al. An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J. Immunol. 155, 5003–5010 (1995).
Constantinescu, C. S. et al. Modulation of susceptibility and resistance to an autoimmune model of multiple sclerosis in prototypically susceptible and resistant strains by neutralization of interleukin-12 and interleukin-4, respectively. Clin. Immunol. 98, 23–30 (2001).
Izikson, L. et al. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192, 1075–1080 (2000).
Glabinski, A. R. et al. Synchronous synthesis of α- and β-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am. J. Pathol. 150, 617–630 (1997).
Okuda, Y. et al. Enhancement of Th2 response in IL-6-deficient mice immunized with myelin oligodendrocyte glycoprotein. J. Neuroimmunol. 105, 120–123 (2000).
Das, M. P., Nicholson, L. B., Greer, J. M. & Kuchroo, V. K. Autopathogenic T helper cell type 1 (Th1) and protective Th2 clones differ in their recognition of the autoantigenic peptide of myelin proteolipid protein. J. Exp. Med. 186, 867–876 (1997).
Boom, W. H., Liano, D. & Abbas, A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J. Exp. Med. 167, 1350–1363 (1988).
Gerritse, K. et al. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl Acad. Sci. USA 93, 2499–2504 (1996).
Chang, T. T. et al. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 190, 733–740 (1999).
Perrin, P. J. et al. Opposing effects of CTLA4-Ig and anti-CD80 (B7-1) plus anti-CD86 (B7-2) on experimental allergic encephalomyelitis. J. Neuroimmunol. 65, 31–39 (1996).
Perrin, P. J. et al. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol. 157, 1333–1336 (1996).
Racke, M. K. et al. Distinct roles for B7–1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J. Clin. Invest. 96, 2195–2203 (1995).
Cross, A. H., Cannella, B., Brosnan, C. F. & Raine, C. S. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab. Invest. 63, 162–170 (1990).
Krakowski, M. L. & Owens, T. The central nervous system environment controls effector CD4+ T cell cytokine profile in experimental allergic encephalomyelitis. Eur. J. Immunol. 27, 2840–2847 (1997).
Ransohoff, R. M., Glabinski, A. & Tani, M. Chemokines in immune-mediated inflammation of the central nervous system. Cytokine Growth Factor Rev. 7, 35–46 (1996).
Aloisi, F., Ria, F., Penna, G. & Adorini, L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J. Immunol. 160, 4671–4680 (1998).
Boddeke, E. W. et al. Cultured rat microglia express functional β-chemokine receptors. J. Neuroimmunol. 98, 176–184 (1999).
McColl, S. R. & Clark-Lewis, I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J. Immunol. 163, 2829–2835 (1999).
Samoilova, E. B. et al. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161, 6480–6486 (1998).
Jacobs, C. A. et al. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J. Immunol. 146, 2983–2989 (1991).
Bourdoulous, S. et al. Anergy induction in encephalitogenic T cells by brain microvessel endothelial cells is inhibited by interleukin-1. Eur. J. Immunol. 25, 1176–1183 (1995).
Özkaynak E et al. Importance of ICOS-B7RP-1 co-stimulation in acute and chronic allograft rejection. Nature Immunol. 2, 591–596 (2001).
Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987).
Sedgwick, J. D. et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl Acad. Sci. USA 88, 7438–7442 (1991).
Acknowledgements
We thank W. Hancock and C. Horvath for critical review of this manuscript and K. McDonald for antibody production and purification.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rottman, J., Smith, T., Tonra, J. et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol 2, 605–611 (2001). https://doi.org/10.1038/89750
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/89750
This article is cited by
-
Ultrasound-responsive spherical nucleic acid against c-Myc/PD-L1 to enhance anti-tumoral macrophages in triple-negative breast cancer progression
Science China Life Sciences (2023)
-
Targeting co-stimulatory molecules in autoimmune disease
Nature Reviews Drug Discovery (2020)
-
T cell-selective deletion of Oct1 protects animals from autoimmune neuroinflammation while maintaining neurotropic pathogen response
Journal of Neuroinflammation (2019)
-
ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells
Scientific Reports (2017)
-
CD3+ICOS+ T cells show differences in the synthesis of nitric oxide, IFN-γ, and IL-10 in patients with pulmonary tuberculosis or in healthy household contacts
Clinical and Experimental Medicine (2016)