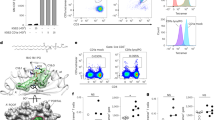
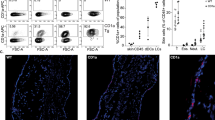
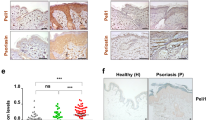
Abstract
CD1a is a lipid-presenting molecule that is abundantly expressed on Langerhans cells. However, the in vivo role of CD1a has remained unclear, principally because CD1a is lacking in mice. Through the use of mice with transgenic expression of CD1a, we found that the plant-derived lipid urushiol triggered CD1a-dependent skin inflammation driven by CD4+ helper T cells that produced the cytokines IL-17 and IL-22 (TH17 cells). Human subjects with poison-ivy dermatitis had a similar cytokine signature following CD1a-mediated recognition of urushiol. Among various urushiol congeners, we identified diunsaturated pentadecylcatechol (C15:2) as the dominant antigen for CD1a-restricted T cells. We determined the crystal structure of the CD1a-urushiol (C15:2) complex, demonstrating the molecular basis of urushiol interaction with the antigen-binding cleft of CD1a. In a mouse model and in patients with psoriasis, CD1a amplified inflammatory responses that were mediated by TH17 cells that reacted to self lipid antigens. Treatment with blocking antibodies to CD1a alleviated skin inflammation. Thus, we propose CD1a as a potential therapeutic target in inflammatory skin diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Brigl, M. & Brenner, M.B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
Porcelli, S. et al. Recognition of cluster of differentiation 1 antigens by human CD4−CD8−cytolytic T lymphocytes. Nature 341, 447–450 (1989).
Kain, L. et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity 41, 543–554 (2014).
Van Rhijn, I., Godfrey, D.I., Rossjohn, J. & Moody, D.B. Lipid and small-molecule display by CD1 and MR1. Nat. Rev. Immunol. 15, 643–654 (2015).
Agea, E. et al. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 202, 295–308 (2005).
Hunger, R.E. et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J. Clin. Invest. 113, 701–708 (2004).
Moody, D.B. et al. T cell activation by lipopeptide antigens. Science 303, 527–531 (2004).
Peña-Cruz, V., Ito, S., Dascher, C.C., Brenner, M.B. & Sugita, M. Epidermal Langerhans cells efficiently mediate CD1a-dependent presentation of microbial lipid antigens to T cells. J. Invest. Dermatol. 121, 517–521 (2003).
Birkinshaw, R.W. et al. αβ T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat. Immunol. 16, 258–266 (2015).
Bourgeois, E.A. et al. Bee venom processes human skin lipids for presentation by CD1a. J. Exp. Med. 212, 149–163 (2015).
de Jong, A. et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat. Immunol. 15, 177–185 (2014).
de Jong, A. et al. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat. Immunol. 11, 1102–1109 (2010).
de Lalla, C. et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur. J. Immunol. 41, 602–610 (2011).
Jarrett, R. et al. Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase. Sci. Transl. Med. 8, 325ra18 (2016).
Zajonc, D.M., Elsliger, M.A., Teyton, L. & Wilson, I.A. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat. Immunol. 4, 808–815 (2003).
Gomez Perdiguero, E. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Greter, M. et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060 (2012).
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760 (2012).
Igyártó, B.Z. & Kaplan, D.H. Antigen presentation by Langerhans cells. Curr. Opin. Immunol. 25, 115–119 (2013).
Merad, M., Ginhoux, F. & Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8, 935–947 (2008).
Bobr, A. et al. Acute ablation of Langerhans cells enhances skin immune responses. J. Immunol. 185, 4724–4728 (2010).
Igyártó, B.Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).
Kaplan, D.H., Igyártó, B.Z. & Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 12, 114–124 (2012).
Lowes, M.A., Bowcock, A.M. & Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 445, 866–873 (2007).
Perera, G.K., Di Meglio, P. & Nestle, F.O. Psoriasis. Annu. Rev. Pathol. 7, 385–422 (2012).
Walker, S.L., Lear, J.T. & Beck, M.H. Toxicodendron dermatitis in the UK. Int. J. Dermatol. 45, 810–813 (2006).
Kalish, R.S. The use of human T-lymphocyte clones to study T-cell function in allergic contact dermatitis to urushiol. J. Invest. Dermatol. 94 (Suppl. 6), S108–S111 (1990).
Kalish, R.S. & Johnson, K.L. Enrichment and function of urushiol (poison ivy)-specific T lymphocytes in lesions of allergic contact dermatitis to urushiol. J. Immunol. 145, 3706–3713 (1990).
Kalish, R.S., Wood, J.A. & LaPorte, A. Processing of urushiol (poison ivy) hapten by both endogenous and exogenous pathways for presentation to T cells in vitro. J. Clin. Invest. 93, 2039–2047 (1994).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Wakabayashi, T. et al. IFN-γ and TNF-α are involved in urushiol-induced contact hypersensitivity in mice. Immunol. Cell Biol. 83, 18–24 (2005).
Illing, P.T. et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 486, 554–558 (2012).
Cai, Y. et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Gray, E.E., Suzuki, K. & Cyster, J.G. Cutting edge: Identification of a motile IL-17-producing γδ T cell population in the dermis. J. Immunol. 186, 6091–6095 (2011).
Kobayashi, C. et al. GM-CSF-independent CD1a expression in epidermal Langerhans cells: evidence from human CD1A genome-transgenic mice. J. Invest. Dermatol. 132, 241–244 (2012).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Stoitzner, P., Romani, N., McLellan, A.D., Tripp, C.H. & Ebner, S. Isolation of skin dendritic cells from mouse and man. Methods Mol. Biol. 595, 235–248 (2010).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Bricogne, G. et al. BUSTER version 2.10.0 (Global Phasing Ltd., Cambridge, UK, 2011).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Alshehry, Z.H. et al. An efficient single phase method for the extraction of plasma lipids. Metabolites 5, 389–403 (2015).
Schittenhelm, R.B., Sian, T.C., Wilmann, P.G., Dudek, N.L. & Purcell, A.W. Revisiting the arthritogenic peptide theory: quantitative not qualitative changes in the peptide repertoire of HLA-B27 allotypes. Arthritis Rheumatol. 67, 702–713 (2015).
Acknowledgements
We thank M. Brenner (Brigham and Women's Hospital, Boston) for anti-CD1a; B. Moody and T.-Y. Cheng (Brigham and Women's Hospital, Boston) for K562 cells and advice on human T cell assays; A. Del Grosso (Food and Drug Administration) for natural urushiols; U. von Andrian and J. Ordovas-Montanes for advice on the preparation of skin tissue; the US National Institutes of Health (NIH) tetramer facility for CD1a monomers; and the staff at the Australian synchrotron for assistance with data collection. Supported by the National Research Foundation of Korea (2012R1A6A3A03040248 to J.H.K.), the AMOREPACIFIC Research Scholar Program (to J.H.K.), National Health and Medical Research Council of Australia (J.R. and A.W.P.), the Australian Research Council (J.R.) and the US National Institutes of Health (R01 AI083426 to F.W.).
Author information
Authors and Affiliations
Contributions
J.H.K. and Y.H. designed and performed experiments and wrote the manuscript; T.Y. performed crystallography and structural analysis; Q.W. and J.K. performed flow cytometry and helped to revise the manuscript; V.A.H., J.L.N., E.A.M. and A.W.P. performed high-performance liquid chromatography, mass spectrometry, and crystallography, and analyzed data; M.S. generated CD1a-tg mice; J.R. and F.W. designed and supervised experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Cytokine pattern in skin in response to urushiol.
Wild-type (WT) and CD1a-tg mice (n = 3 per group) were sensitized and challenged with urushiol (uru) or vehicle (veh). Quantitative real-time PCR was performed to analyze cytokine gene expression in the ear tissues obtained on day 2 after challenge. Results are presented as fold increases over vehicle-treated ear tissues from wild-type mice. Results are representative of two independent experiments (mean ± s.e.m).
Supplementary Figure 2 CD1a suppresses contact hypersensitivity mediated by IFN-γ-producing cells.
Wild-type (WT) and CD1a-tg mice (n = 3 per group) were sensitized with 0.5% DNFB by painting on the abdomen on day 0, and challenged with either 0.2% DNFB or vehicle (veh) on ears on day 5 after sensitization. (a) Ear swelling after challenge. (b) Frequencies of Gr-1hiCD11bhi granulocytes (left panel), CD4+ or CD8+ T cells among live CD45+TCRβ+ cells (middle panel), and IL-17A+ or IFN-γ+ cells among TCRβ+ cells (right panel). (c) Absolute cell numbers of CD8+ or CD4+ ab T cells, IFN-γ- or IL-17A-producing cells in ears. * P < 0.05, ** P < 0.01, *** P < 0.001, using unpaired t-test. Results are representative of three independent experiments (mean ± s.e.m).
Supplementary Figure 3 Vβ TCR subfamily profile in skin and thymus.
(a-e) Ear cells or thymocytes were isolated from wild-type (WT) or CD1a-tg mice on day 2 after urushiol C15:2 or vehicle challenge and analyzed for 15 different Vβ TCR subfamilies using flow cytometry. Relative contribution of each Vβ+ cell subset among CD45+CD3+CD4+ T cells is presented in bar graphs. (a) Vβ TCR repertoire in urushiol-treated skin. (b) Frequencies of IL-17A+ cells among Vβ2+ or Vβ4+ CD4+ T cells in ear. (c) Frequencies of Vβ2+ or Vβ4+ cells in ears from CD1a-tg versus CD1a-tg mice injected with anti-CD1a antibody. (d) Vβ TCR repertoire in vehicle-treated skin. (e) Vβ TCR repertoire in thymus. * P < 0.05, ** P < 0.01; NS, not significant, using unpaired t-test. Results are representative of two to three independent experiments (mean ± s.e.m, a,c,d).
Supplementary Figure 4 Electron-density ‘shots’ of urushiol in the antigen-binding cleft of CD1a.
(a) Fo-Fc electron density map (yellow orange) of urushiol contoured at 2.2 σ level. (b) 2Fo-Fc electron density map (blue) of urushiol contoured at 0.8 σ level.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1–4 (PDF 1435 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Hu, Y., Yongqing, T. et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat Immunol 17, 1159–1166 (2016). https://doi.org/10.1038/ni.3523
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3523
This article is cited by
-
Human umbilical cord mesenchymal stem cell treatment alleviates symptoms in an atopic dermatitis-like mouse model
Stem Cell Research & Therapy (2023)
-
The emerging scenario of immunotherapy for T-cell Acute Lymphoblastic Leukemia: advances, challenges and future perspectives
Experimental Hematology & Oncology (2023)
-
The IL-17 family in diseases: from bench to bedside
Signal Transduction and Targeted Therapy (2023)
-
IL-17 and IL-17-producing cells in protection versus pathology
Nature Reviews Immunology (2023)
-
CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system
Experimental & Molecular Medicine (2023)