Abstract
Group 2 innate lymphoid cells (ILC2s) regulate tissue inflammation and repair after activation by cell-extrinsic factors such as host-derived cytokines. However, the cell-intrinsic metabolic pathways that control ILC2 function are undefined. Here we demonstrate that expression of the enzyme arginase-1 (Arg1) during acute or chronic lung inflammation is a conserved trait of mouse and human ILC2s. Deletion of mouse ILC-intrinsic Arg1 abrogated type 2 lung inflammation by restraining ILC2 proliferation and dampening cytokine production. Mechanistically, inhibition of Arg1 enzymatic activity disrupted multiple components of ILC2 metabolic programming by altering arginine catabolism, impairing polyamine biosynthesis and reducing aerobic glycolysis. These data identify Arg1 as a key regulator of ILC2 bioenergetics that controls proliferative capacity and proinflammatory functions promoting type 2 inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
McKenzie, A.N., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014).
Chang, Y.J. et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12, 631–638 (2011).
Barlow, J.L. et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129, 191–198 (2012).
Halim, T.Y. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
Monticelli, L.A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011).
Buck, M.D., O'Sullivan, D. & Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 212, 1345–1360 (2015).
Vander Heiden, M.G., Cantley, L.C. & Thompson, C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Bando, J.K., Nussbaum, J.C., Liang, H.E. & Locksley, R.M. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J. Leukoc. Biol. 94, 877–884 (2013).
Bando, J.K., Liang, H.E. & Locksley, R.M. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat. Immunol. 16, 153–160 (2015).
Rath, M., Muller, I., Kropf, P., Closs, E.I. & Munder, M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 5, 532 (2014).
Thomas, A.C. & Mattila, J.T. “Of mice and men”: arginine metabolism in macrophages. Front. Immunol. 5, 479 (2014).
Murray, P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 17, 132–139 (2015).
Raber, P., Ochoa, A.C. & Rodriguez, P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol. Invest. 41, 614–634 (2012).
Wynn, T.A., Chawla, A. & Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Murray, P.J. & Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Gibbons, M.A. et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 184, 569–581 (2011).
Rodriguez, P.C., Quiceno, D.G. & Ochoa, A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573 (2007).
Pesce, J.T. et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371 (2009).
Barron, L. et al. Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS ONE 8, e61961 (2013).
Cloots, R.H. et al. Ablation of Arg1 in hematopoietic cells improves respiratory function of lung parenchyma, but not that of larger airways or inflammation in asthmatic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L364–L376 (2013).
North, M.L., Khanna, N., Marsden, P.A., Grasemann, H. & Scott, J.A. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L911–L920 (2009).
Yang, M. et al. Inhibition of arginase I activity by RNA interference attenuates IL-13-induced airways hyperresponsiveness. J. Immunol. 177, 5595–5603 (2006).
Hoyler, T. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012).
Lara, A. et al. Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care Med. 178, 673–681 (2008).
Bratt, J.M., Zeki, A.A., Last, J.A. & Kenyon, N.J. Competitive metabolism of L-arginine: arginase as a therapeutic target in asthma. J. Biomed. Res. 25, 299–308 (2011).
Holguin, F. et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am. J. Respir. Crit. Care Med. 187, 153–159 (2013).
Vonk, J.M. et al. Arginase 1 and arginase 2 variations associate with asthma, asthma severity and β2 agonist and steroid response. Pharmacogenet. Genomics 20, 179–186 (2010).
Bergeron, C. et al. Influence of cigarette smoke on the arginine pathway in asthmatic airways: increased expression of arginase I. J. Allergy Clin. Immunol. 119, 391–397 (2007).
Tadié, J.M. et al. Role of nitric oxide synthase/arginase balance in bronchial reactivity in patients with chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L489–L497 (2008).
Wu, G. et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37, 153–168 (2009).
Hams, E. et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 111, 367–372 (2014).
Turner, J.E. et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 210, 2951–2965 (2013).
Wojno, E.D. et al. The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 8, 1313–1323 (2015).
Reece, J.J., Siracusa, M.C. & Scott, A.L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect. Immun. 74, 4970–4981 (2006).
Marsland, B.J., Kurrer, M., Reissmann, R., Harris, N.L. & Kopf, M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 38, 479–488 (2008).
Fujita, M. & Nakanishi, Y. The pathogenesis of COPD: lessons learned from in vivo animal models. Med. Sci. Monit. 13, RA19–RA24 (2007).
Demkow, U. & van Overveld, F.J. Role of elastases in the pathogenesis of chronic obstructive pulmonary disease: implications for treatment. Eur. J. Med. Res. 15, 27–35 (2010).
Sonnenberg, G.F., Monticelli, L.A., Elloso, M.M., Fouser, L.A. & Artis, D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134 (2011).
Knippenberg, S., Brumshagen, C., Aschenbrenner, F., Welte, T. & Maus, U.A. Arginase 1 activity worsens lung-protective immunity against Streptococcus pneumoniae infection. Eur. J. Immunol. 45, 1716–1726 (2015).
Miller-Fleming, L., Olin-Sandoval, V., Campbell, K. & Ralser, M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 427, 3389–3406 (2015).
Phang, J.M., Liu, W., Hancock, C.N. & Fischer, J.W. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr. Opin. Clin. Nutr. Metab. Care 18, 71–77 (2015).
Millard, P., Letisse, F., Sokol, S. & Portais, J.C. IsoCor: correcting MS data in isotope labeling experiments. Bioinformatics 28, 1294–1296 (2012).
van der Windt, G.J. & Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 249, 27–42 (2012).
Molofsky, A.B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Li, D. et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 134, 1422–1432 (2014).
Xue, L. et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 133, 1184–1194 (2014).
Schlenner, S.M. et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32, 426–436 (2010).
Brestoff, J.R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Osborne, L.C. et al. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science 345, 578–582 (2014).
Acknowledgements
We thank members of the D. Artis and G.F. Sonnenberg laboratories for critical reading of this manuscript. We thank R.M. Locksley (University of California, San Francisco, San Francisco, California, USA) for generously providing the Arg1YFP mice and B. Vallance (University of British Columbia, Vancouver, British Columbia, Canada) for providing Citrobacter rodentium. This work was supported by the National Institutes of Health (NIH) (grants AI061570, AI087990, AI074878, AI083480, AI095466, AI095608, AI102942 and AI097333 to D.A.; T32-AI007532 to L.A.M.; CA181125 and AI091965 to E.L.P.; HL087115, HL081619, HL096845, HL115354 and HL114626 to J.D.C.; HL116656 to E.C.; and HL090021 and K23-HL121406 to J.M.D.), the Burroughs Wellcome Fund (D.A. and E.L.P.), the Crohn's & Colitis Foundation of America (D.A. and M.R.H.), the Edmond J. Safra Foundation/Cancer Research Institute (L.C.O.), the National Science Foundation (grant DGE-1143954 to M.D.B.), the Robert Wood Johnson Foundation (grant AMFDP 70640 to E.C.), the Thoracic Surgery Foundation (E.C.) and the German Research Foundation (DFG SFB 873–project 11 to H.-R.R.). We thank the Mucosal Immunology Studies Team (MIST) of the NIH NIAID for shared expertise and resources.
Author information
Authors and Affiliations
Contributions
L.A.M. carried out mouse experiments with technical assistance from A.-L.F., N.A.Y., L.C.O., M.R.H. and S.V.T. M.D.B. and E.L.P. carried out Seahorse metabolic assays. H.S. and J.R.C. carried out liquid chromatography–mass spectrometry experiments. J.M.D., E.C. and J.D.C. provided human tissue samples. L.A.M., S.A.S. and E.D.T.W. carried out experiments involving human tissue samples. H.-R.R. provided Il7rCre/+ mice. L.A.M. and D.A. conceived of the study, designed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
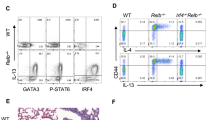
Supplementary Figure 1 Distribution of human ILCs during chronic lung disease.
Quantification of flow cytometric analysis of lung tissue from patients with COPD or IPF identifying (a) frequencies of total Lin−CD127+ ILCs (Lin gating includes CD3, CD5, CD56, CD19, FcεR1, CD14, CD16). Cells are pregated on live CD45+ cells. (b) Frequency of ILC subsets defined by IL-33R (ST2L) and CRTH2 expression, pregated on Lin−CD127+ cells. (c) Mean fluorescence intensity (MFI) of intracellular Arg1 expression in each cell subset. Data shown are from one experiment. Data are the mean ± SEM, n = 8 COPD and n = 8 IPF tissue samples.
Supplementary Figure 2 Fate-mapping analysis of IL-7Rα expression in immune cell subsets during lung inflammation.
Il7rcre/+ Rosa26floxSTOP-eYFP and C57BL/6J WT mice were treated with 30 μg papain or PBS intranasally (i.n.) for 5 days and analyzed on day 6. Representative flow cytometric histograms of eYFP expression from lung immune cell subsets, Il7rcre/+ Rosa26floxSTOP-eYFP red line; WT, gray shaded. Numbers indicate the percentage of total cells that are marked by a history of IL-7Rα expression for ILC2s (Lin−CD45+CD90+CD25+IL-33R+), CD4+ T cells (CD3+CD4+), B cells (CD19+CD3−), NK cells (NK1.1+CD3−) and eosinophils (CD11b+Siglec F+CD11c−). All data are representative of two independent experiments with similar results. N = 3-4 mice per group.
Supplementary Figure 3 Analysis of Arg1 expression in immune cell subsets during lung inflammation.
Arg1YFP and C57BL/6J WT mice were treated with 30 μg papain or PBS i.n. for 5 days and analyzed on day 6. Representative flow cytometric histograms of Arg1-YFP expression from lung immune cell subsets, Arg1YFP, blue line; WT, gray shaded. Numbers indicate the percentage of total cells that express Arg1 for ILC2s (Lin−CD45+CD90+CD25+IL-33R+), CD4+ T cells (CD3+CD4+), B cells (CD19+CD3−), NK cells (NK1.1+CD3−) and eosinophils (CD11b+Siglec F+CD11c−). All data are representative of two independent experiments with similar results. N = 3-4 mice per group.
Supplementary Figure 4 Loss of ILC-intrinsic Arg1 impairs ILC2 responses during chronic lung inflammation.
(a-d) Arg1fl/fl and Arg1ΔILC mice were infected with 500 L3 Nippostrongylus brasiliensis (Nb) larvae subcutaneously and assessed one month post infection. (a) Representative flow cytometric plots and (b) total frequencies of ILC2s (Lin−CD45+CD90+CD25+) in the lungs of naïve and Nb-infected mice. (c) mRNA expression of (c) Arg1 in lung tissue, determined by RT-PCR and expressed relative to levels in naive Arg1fl/fl mice. (d-g) Arg1fl/fl and Arg1ΔILC mice were instilled with 3 units of elastase or PBS intratracheally and assessed one month post treatment. (d) Representative flow cytometric plots and (e) total frequencies of ILC2s (Lin− CD45+ CD90+ CD25+) in the lungs of PBS and elastase-treated mice. (f) mRNA expression of Arg1 in lung tissue, determined by RT-PCR and expressed relative to levels in PBS-treated Arg1fl/fl mice. (g) Representative histological sections of whole left lung lobes stained with H&E. Scale bar, 100 μm. Data are representative of two independent experiments with similar results. N = 2 mice (PBS and naïve) and n = 4 mice (Nb and elastase). Data shown are the mean ± SEM. *p < 0.05, **p < 0.01, *** p < 0.001, as determined by unpaired Student’s t test.
Supplementary Figure 5 ILC3 development and anti-bacterial immunity are independent of ILC-intrinsic Arg1.
(a) Representative flow cytometric plots and (b) frequencies of total ILCs (Lin−CD45+CD90+CD25+) in the gut-associated mesenteric lymph nodes (mLN) of naïve Arg1fl/fl and Arg1ΔILC mice. (c) Representative flow cytometric plots and (d) total frequencies of GATA3hi ILC2 versus RORγt+ ILC3 populations, parent gate is total ILCs as in (a,b). (e) RORc(γt)gfp/gfp, Rag2-/-Il2rg-/-, Rag1-/-, Arg1fl/fl and Arg1ΔILC mice were infected with 1 × 1010 CFU of Citrobacter rodentium (C. rodentium) in 200 µL via oral gavage. Mice were monitored for morbidity and mortality over the course of 28 days. Data are representative of two independent experiments with similar results. N = 2-5 mice per group. Data shown are the mean ± SEM. NS, not significant.
Supplementary Figure 6 ILC-intrinsic Arg1 does not influence cell survival.
(a-c) Arg1fl/fl, Arg1+/+ Il7rCre/+ and Arg1ΔILC were treated with 30 μg papain or PBS i.n. for 5 days and assessed on day 6 for survival of lung ILC2s by Annexin V and 7AAD staining. (a) Representative flow cytometric plot showing validation of gating strategy to identify dead cells by 7AAD and Annexin V co-staining. (b) Representative flow cytometric plots and (c) quantification of Annexin V+ 7AAD+ ILC2 frequencies. ILC2s gated as Lin−CD45+CD90+CD25+. (d) C57BL/6J WT mice were treated with 100 ng of rmIL-33 intranasally every 3 days for 2 weeks. Sort-purified lung ILC2s (Lin−CD45+CD90+CD127+CD25+IL-33R+) were labeled with cell trace violet and cultured for 48 h with rmIL-2, rmIL-7, rmIL-33 and DMSO or Nω-hydroxy-nor-Arginine (nor-NOHA). Cells were assessed for dilution of the cell trace dye using flow cytometry. Data are representative of two experiments (a-c) or three experiments (d) with similar results. N = 3-4 mice per group.
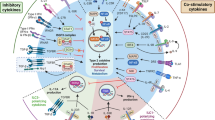
Supplementary Figure 7 ILC-intrinsic arginase metabolism.
(a) Pathway diagram of arginine metabolism in activated ILC2s. Green indicates positive metabolism, black indicates no significant metabolism, grey indicates metabolite not detected or not examined. Blue indicates enzymes arginase (Arg1), nitric oxide synthase (NOS) and L-Arginine:glycine amidinotransferase (AGAT). (b) Experimental schematic for liquid chromatography mass spectrometry analysis. C57BL/6J WT mice were treated with 100 ng rmIL-33 i.n. every 3 days for 2-3 weeks and sort-purified lung ILC2s (Lin−CD45+CD90+CD127+CD25+IL-33R+) were cultured with rmIL-2, rmIL-7 and rmIL-33 in DMSO or 500μM nor-NOHA for 24 h. Methanol-fixed cells were subjected to liquid chromatography mass spectrometry analysis.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 2731 kb)
Rights and permissions
About this article
Cite this article
Monticelli, L., Buck, M., Flamar, AL. et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17, 656–665 (2016). https://doi.org/10.1038/ni.3421
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3421
This article is cited by
-
Shape morphing of hydrogels by harnessing enzyme enabled mechanoresponse
Nature Communications (2024)
-
Identification of potential biomarkers and therapeutic targets for posttraumatic acute respiratory distress syndrome
BMC Medical Genomics (2023)
-
Arginase-1 inhibition reduces migration ability and metastatic colonization of colon cancer cells
Cancer & Metabolism (2023)
-
The modulation of pulmonary group 2 innate lymphoid cell function in asthma: from inflammatory mediators to environmental and metabolic factors
Experimental & Molecular Medicine (2023)
-
Proline fuels ILC3s to maintain gut health
Nature Metabolism (2023)