Abstract
Innate lymphoid cells (ILCs) 'preferentially' localize into barrier tissues, where they function in tissue protection but can also contribute to inflammatory diseases. The mechanisms that regulate the establishment of ILCs in barrier tissues are poorly understood. Here we found that under steady-state conditions, ILCs in skin-draining lymph nodes (sLNs) were continuously activated to acquire regulatory properties and high expression of the chemokine receptor CCR10 for localization into the skin. CCR10+ ILCs promoted the homeostasis of skin-resident T cells and, reciprocally, their establishment in the skin required T cell–regulated homeostatic environments. CD207+ dendritic cells expressing the transcription factor Foxn1 were required for the proper generation of CCR10+ ILCs. These observations reveal mechanisms that underlie the specific programming and priming of skin-homing CCR10+ ILCs in the sLNs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Spits, H. et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Walker, J.A., Barlow, J.L. & McKenzie, A.N. Innate lymphoid cells–how did we miss them? Nat. Rev. Immunol. 13, 75–87 (2013).
Monticelli, L.A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573 (2013).
Hepworth, M.R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
Nussbaum, J.C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014).
Powell, N. et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 37, 674–684 (2012).
Geremia, A. et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208, 1127–1133 (2011).
Coccia, M. et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med. 209, 1595–1609 (2012).
Wilhelm, C. et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12, 1071–1077 (2011).
Chang, Y.J. et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12, 631–638 (2011).
Kim, H.Y. et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 129, 216–227 (2012).
Kim, H.Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 20, 54–61 (2014).
Kim, B.S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra116 (2013).
Salimi, M. et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 210, 2939–2950 (2013).
Imai, Y. et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl. Acad. Sci. USA 110, 13921–13926 (2013).
Pantelyushin, S. et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 (2012).
Mjösberg, J.M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12, 1055–1062 (2011).
Constantinides, M.G., McDonald, B.D., Verhoef, P.A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Hoyler, T. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012).
Homey, B. et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J. Immunol. 164, 3465–3470 (2000).
Xia, M. et al. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J. Allergy Clin. Immunol. 134, 634–644 e610 (2014).
Jin, Y., Xia, M., Sun, A., Saylor, C.M. & Xiong, N. CCR10 is important for the development of skin-specific γδT cells by regulating their migration and location. J. Immunol. 185, 5723–5731 (2010).
Spencer, S.P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
Eberl, G. et al. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Sigmundsdottir, H. et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 8, 285–293 (2007).
Kiss, E.A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Rennert, P.D., Browning, J.L., Mebius, R., Mackay, F. & Hochman, P.S. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184, 1999–2006 (1996).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Neill, D.R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Nehls, M. et al. Two genetically separable steps in the differentiation of thymic epithelium. Science 272, 886–889 (1996).
Flanagan, S.P. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 8, 295–309 (1966).
Kissenpfennig, A. et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654 (2005).
Bobr, A. et al. Acute ablation of Langerhans cells enhances skin immune responses. J. Immunol. 185, 4724–4728 (2010).
McCully, M.L. et al. Epidermis instructs skin homing receptor expression in human T cells. Blood 120, 4591–4598 (2012).
Sigmundsdottir, H. & Butcher, E.C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 9, 981–987 (2008).
Moed, H. et al. Increased CCL27–CCR10 expression in allergic contact dermatitis: implications for local skin memory. J. Pathol. 204, 39–46 (2004).
Kakinuma, T. et al. Increased serum cutaneous T cell-attracting chemokine (CCL27) levels in patients with atopic dermatitis and psoriasis vulgaris. J. Allergy Clin. Immunol. 111, 592–597 (2003).
Gudjonsson, J.E. et al. Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J. Invest. Dermatol. 130, 1829–1840 (2010).
Riis, J.L. et al. Kinetics and differential expression of the skin-related chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Exp. Dermatol. 20, 789–794 (2011).
Hu, S., Yang, K., Yang, J., Li, M. & Xiong, N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc. Natl. Acad. Sci. USA 108, E1035–E1044 (2011).
Li, M. et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 103, 11736–11741 (2006).
Acknowledgements
We thank C. Bradfield (University of Wisconsin) for Ahr−/− mice; and X. Zang (Albert Einstein College of Medicine) for antibodies to mouse PDL-2, mouse B7x, mouse B7h and mouse B7H3. Supported by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis, Musculoskeletal and Skin Diseases and National Institute of Environmental Health Sciences of the US National Institutes of Health (R01AI071043 and R01AR064831 to N.X., and ES019964 to G.H.P.) and matching institutional funds from Pennsylvania State University for T32 AI074551 from the National Institutes of Health (for J.Y.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.Y., S.H. and L.Z. performed experiments; D.H.K. and G.H.P. provided materials; J.Y. and N.X. designed the study; J.Y., S.H., L.Z. and N.X. analyzed the data and wrote the manuscript; and all read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
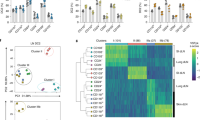
Supplementary Figure 1 Characterization of CCR10+ and CCR10– ILCs of the skin and sLNs.
(a) Flow cytometric analysis of Gata-3 and T-bet expression in EGFP(CCR10)+ and EGFP– CD45+CD3–Lin– ILCs of the skin and sLN of Ccr10+/EGFP mice. (b) Flow cytometric analysis of Rorγt expression in CD45+CD3–Lin– ILCs of the skin and sLN of mice carrying one Rorc-EGFP allele for reporting Rorγt expression. The gray histogram is of wild-type ILCs as a negative control (-CTRL) for Rorγt-EGFP. The Rorγt expression in γdT cells is included as a positive control. (c) Comparison of average median fluorescence intensity (MFI) of the MHCII staining of EGFP+MHCII+ vs. EGFP–MHCII+ CD45+CD3–Lin– skin ILCs of Ccr10+/EGFP mice. N=5. (d) Flow cytometric analysis of co-expression of MHCII and IL-17 or IL-5 in CCR10+ CD45+CD3–Lin– ILCs of the sLN and skin of Ccr10+/EGFP mice. (e and f) Flow cytometric analysis of expression of indicated co-inhibitory and co-stimulatory molecules on gated EGFP(CCR10)+ CD45+CD3–Lin– ILCs in the skin (e) and sLN (f) of Ccr10+/EGFP mice. Gray histograms are of the staining with isotype control antibodies. **P<0.01.
Supplementary Figure 2 Homeostatic establishment of CCR10+ ILCs in the skin depends on continuous input of CCR10+MHCII+ ILCs from sLNs.
(a) Comparison of average numbers of EGFP+ CD45+CD3–Lin– ILCs in the sLN and skin of FTY720 (FTY)-treated and untreated (CTRL) Ccr10+/EGFP mice. Analyzed 2 weeks after the treatment. N=3. (b) Analysis of T cells of sLN and the skin for their expression of CCR10 (EGFP) in FTY720 (FTY)-treated and untreated (CTRL) Ccr10+/EGFP mice. Analyzed 2 weeks after the treatment. Bar graphs show average percentages and numbers of EGFP+ sub-populations of total T cells in the sLN and skin. N=3. (c) Comparison of numbers of CCR10 (EGFP)+ CD45+CD3–Lin– ILCs in the skin of Ccr10+/EGFP (WT), Rorc–/–Ccr10+/EGFP (Rorc–/–) and Ahr–/–Ccr10+/EGFP (Ahr–/–) mice. N=3. (d) Flow cytometric analysis of expression of CCR10 (EGFP) on CD3int skin T cells of Ccr10+/EGFP (WT) and Rorc–/–Ccr10+/EGFP (Rorc–/–) mice. (e) Flow cytometric analysis of expression of IL-17 and IL-5 on skin CD45+CD3–Lin– ILCs of Ccr10+/EGFP (WT) and Rorc–/–Ccr10+/EGFP (Rorc–/–) mice. (f) Flow cytometric analysis of expression of CCR10 (EGFP) and other indicated molecules on CD45+CD3–Lin– ILCs of sLN and the skin of Ahr–/–Ccr10+/EGFP mice. (g-h) Flow cytometric analysis of expression of CCR10 (EGFP) and IL-17 and IL-5 on CD45+CD3–Lin– ILCs of sLN (g) and the skin (h) of Ahr–/–Ccr10+/EGFP (Ahr–/–) and Ccr10+/EGFP (WT) mice. (i) Flow cytometric analysis of expression of CCR10 (EGFP) on CD3int skin T cells of Ccr10+/EGFP (WT) and Ahr–/–Ccr10+/EGFP (Ahr–/–) mice. (j) Comparison of average numbers of EGFP(CCR10)+ and EGFP+MHCII+ CD45+CD3–Lin– ILCs in the skin of Ccr10+/EGFP mice treated with LTβR-Ig in utero and untreated Ccr10+/EGFP control (CTRL) mice. N=6 for control and 10 for LTβR-Ig-treated mice. *P<0.05, **P<0.01, ***P<0.001, NS: no significant difference.
Supplementary Figure 3 Characterization of ILCs in Ccr6+/–Ccr10+/EGFP, Ccr6+/–Ccr10EGFP/EGFP, Ccr6–/–Ccr10+/EGFP and Ccr6–/–Ccr10EGFP/EGFP mice.
(a) Comparison of numbers of EGFP(CCR10)+ CD45+CD3–Lin– ILCs in sLN, skin and spleens of Ccr6+/–Ccr10+/EGFP, Ccr6+/–Ccr10EGFP/EGFP, Ccr6–/–Ccr10+/EGFP, and Ccr6–/–Ccr10EGFP/EGFP mice. N=5. (b) Flow cytometric identification of CD45+CD3–Lin– ILCs in the skin of Ccr6+/–Ccr10+/EGFP, Ccr6+/–Ccr10EGFP/EGFP, Ccr6–/–Ccr10+/EGFP, and Ccr6–/–Ccr10EGFP/EGFP mice. Gated on CD45+ lymphocytes. The bar graph on the right shows average percentages of ILCs in total CD45+ lymphocytes. N=4. (c) Flow cytometric analysis of expression of CCR10 (EGFP) and other indicated molecules on CD45+CD3–Lin– ILCs from sLN and skin of Ccr10EGFP/EGFP mice. Performed twice. (d) Representative flow cytometric analysis of donor Ccr10+/EGFP and Ccr10EGFP/EGFP EGFP+ CD45+CD3–Lin– sLN ILCs recovered in the skin of wild-type recipients 2 days after transfer of similar numbers of Ccr10+/EGFP (+/-) (CD45.1+CD45.2+) and Ccr10EGFP/EGFP (-/-) (CD45.1–CD45.2+) EGFP+ sLN ILCs (After). sLN ILCs were labeled with Violet dye before transfer for identification. Gated on Violet+45.2+CD3–Lin– EGFP+ cells. The mixture of donor sLN ILCs before transfer (Before) was shown as a control. *P<0.05, **P<0.01, NS: no significant difference.
Supplementary Figure 4 Different programming and maintenance of CCR10+ and CCR10– ILCs in sLNs and the skin in homeostatic and inflammatory conditions.
(a) Comparison of numbers of EGFP (CCR10)+ CD45+CD3–Lin– ILCs in the skin of Ccr10+/EGFP (WT) and Rag1–/–Ccr10+/EGFP (Rag1-/-) mice. N=5. (b) Flow cytometric analysis of expression of CCR10 (EGFP) and other indicated molecules on CD45+CD3–Lin– ILCs from sLN and skin of Rag1–/–Ccr10+/EGFP mice. N=3. (c) Flow cytometric analysis of expression of IL-17 and IL-5 on CD45+CD3–Lin– ILCs in the skin of Ccr10+/EGFP (WT) and Rag1–/–Ccr10+/EGFP (Rag1–/–) mice. (d) Flow cytometric analysis of donor CD45+CD3–Lin– ILCs and their expression of CCR10 and MHCII in the skin of Rag1–/– (CD45.1+CD45.2+) mice that received transfer of total sLN cells of Rag1–/–Ccr10+/EGFP (CD45.2+) mice or sorter-purified EGFP (CCR10)+ CD45+CD3–Lin– sLN ILCs of Ccr10+/EGFP (WT) or Rag1–/–Ccr10+/EGFP (Rag1–/–) (CD45.2+) mice. Analyzed two days or four weeks after transfer. Representative of two experiments. (e) Flow cytometric analysis of expression of CD44 and MHCII on EGFP+ vs. EGFP– CD45+CD3–Lin– host ILCs from sLN of Rag1–/–Ccr10+/EGFP mice transferred with Ccr10+/EGFP CD4+ splenic T cells. Analyzed 6 weeks after transfer. (f) Comparison of average numbers of EGFP(CCR10)+ CD45+CD3–Lin– ILCs in the sLN and skin of Ccr10+/EGFP (WT) and Foxp3–/–Ccr10+/EGFP (Foxp3–/–) mice. N=4. (g) Comparison of average numbers of EGFP(CCR10)+ CD45+CD3–Lin– ILCs in sLNs and the skin of calcipotriol-treated (Calcipotriol, N=7) versus untreated (CTRL, N=6) Ccr10+/EGFP mice. *P<0.05, **P<0.01.
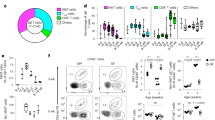
Supplementary Figure 5 CCR10+ ILCs regulate the homeostasis of resident helper T cells in the skin.
(a) Flow cytometric analysis of donor CD4+ T cells and their expression of IL-17, IFN-γ and IL-5 in the skin of Rag1–/– and Rag1–/–Ccr10EGFP/EGFP recipient mice that were transferred with total CD4+ splenic T cells from Ccr10+/EGFP mice. Bar graphs show average percentages of IL-17, IFN-g and IL-5-producing CD4+ T cells of the donor T cells in the skin of Rag1–/– and Rag1–/–Ccr10EGFP/EGFP recipients. N=3. NS: no significant difference.
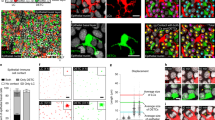
Supplementary Figure 6 Transcription factor Foxn1 and CD207+ dendritic cells are involved in the programming of CCR10+ ILCs.
(a) Comparison of average numbers of EGFP(CCR10)+ CD45+CD3–Lin– ILCs in the sLN and skin of Ccr10+/EGFP (WT) and Foxn1–/–Ccr10+/EGFP (Foxn1–/–) mice. N=4. (b) Flow cytometric analysis of EGFP+ and EGFP– CD45+CD3–Lin– ILCs of Ccr10+/EGFP donor BM cells in the skin of Foxn1–/– vs. wild-type recipient mice (CD45.2+) that were irradiated and transferred with similar numbers of total BM cells from Ccr10+/EGFP (CD45.1+CD45.2+) mice. Analyzed eight weeks after transfer. Bar graphs show average percentages of EGFP+ and EGFP+MHCII+ subpopulations of total donor cell-derived CD45+CD3–Lin– ILCs in the skin of recipients. N=5 for wild-type and 4 for Foxn1–/– mice. (c) Flow cytometric analysis of Foxn1 (RFP) expression in CD103+ and CD103– CD207+CD45+CD3–CD11c+ DCs of the sLN and skin of Foxn1+/CreGt(ROSA)26Sor+/RFP mice. Cells of Foxn1+/+Gt(ROSA)26SorRFP/RFP mice were included as negative control for RFP signals. (d) Flow cytometric analysis of CD45+CD3–Lin– ILCs and their EGFP and MHCII expression in sLNs and the skin of diphtheria toxin (DT)-treated and untreated (CTRL) MuLanDTRCcr10+/EGFP mice. Analyzed 8 days after DT treatment. (e) Comparison of average percentages of EGFP+ T cells in sLNs and the skin of MuLanDTRCcr10+/EGFP mice treated with DT for indicated periods of time. CTRL: untreated controls. N=5 for CTRL and 7 for DT for the 8-day sLN samples, 3 for CTRL and 6 for DT for the 17-21-day sLN and skin samples. (f) Comparison of percentages of EGFP+ T cells in sLNs and the skin of DT-treated HuLanDTRCcr10+/EGFP mice, analyzed similarly as in the panel (e). N=7 for CTRL and 6 for DT for the 8-day sLN samples, 4 each for the 17-21-day sLN and skin samples. *P<0.05, **P<0.01, ***P<0.001, NS: no significant difference.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1455 kb)
Supplementary Table 1
Excel table of antibodies (XLS 47 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Hu, S., Zhao, L. et al. Selective programming of CCR10+ innate lymphoid cells in skin-draining lymph nodes for cutaneous homeostatic regulation. Nat Immunol 17, 48–56 (2016). https://doi.org/10.1038/ni.3312
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3312
This article is cited by
-
Developmentally programmed early-age skin localization of iNKT cells supports local tissue development and homeostasis
Nature Immunology (2023)
-
New genetic and epigenetic insights into the chemokine system: the latest discoveries aiding progression toward precision medicine
Cellular & Molecular Immunology (2023)
-
Dynamic regulation of innate lymphoid cells in the mucosal immune system
Cellular & Molecular Immunology (2021)
-
Identification of potential key mRNAs and LncRNAs for psoriasis by bioinformatic analysis using weighted gene co-expression network analysis
Molecular Genetics and Genomics (2020)
-
Signs of innate immune activation and premature immunosenescence in psoriasis patients
Scientific Reports (2017)