Abstract
The thymic production of regulatory T cells (Treg cells) requires interleukin 2 (IL-2) and agonist T cell antigen receptor (TCR) ligands and is controlled by competition for a limited developmental niche, but the thymic sources of IL-2 and the factors that limit access to the niche are poorly understood. Here we found that IL-2 produced by antigen-bearing dendritic cells (DCs) had a key role in Treg cell development and that existing Treg cells limited new development of Treg cells by competing for IL-2. Our data suggest that antigen-presenting cells (APCs) that can provide both IL-2 and a TCR ligand constitute the thymic niche and that competition by existing Treg cells for a limited supply of IL-2 provides negative feedback for new production of Treg cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184, 387–396 (1996).
Itoh, M. et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162, 5317–5326 (1999).
Sawant, D.V. & Vignali, D.A. Once a Treg, always a Treg? Immunol. Rev. 259, 173–191 (2014).
Riley, J.L., June, C.H. & Blazar, B.R. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30, 656–665 (2009).
von Boehmer, H. & Daniel, C. Therapeutic opportunities for manipulating TReg cells in autoimmunity and cancer. Nat. Rev. Drug Discov. 12, 51–63 (2013).
Burchill, M.A. et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28, 112–121 (2008).
Lio, C.W. & Hsieh, C.S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Moran, A.E. & Hogquist, K.A. T-cell receptor affinity in thymic development. Immunology 135, 261–267 (2012).
Jordan, M.S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001).
Bautista, J.L. et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10, 610–617 (2009).
Leung, M.W., Shen, S. & Lafaille, J.J. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J. Exp. Med. 206, 2121–2130 (2009).
Granucci, F. et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2, 882–888 (2001).
Kulhankova, K., Rouse, T., Nasr, M.E. & Field, E.H. Dendritic cells control CD4+CD25+ Treg cell suppressor function in vitro through juxtacrine delivery of IL-2. PLoS ONE 7, e43609 (2012).
Vang, K.B. et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181, 3285–3290 (2008).
Bayer, A.L., Yu, A., Adeegbe, D. & Malek, T.R. Essential role for interleukin-2 for CD4+ CD25+ T regulatory cell development during the neonatal period. J. Exp. Med. 201, 769–777 (2005).
Fontenot, J.D., Rasmussen, J.P., Gavin, M.A. & Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Lee, H.M., Bautista, J.L., Scott-Browne, J., Mohan, J.F. & Hsieh, C.S. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity 37, 475–486 (2012).
Atibalentja, D.F., Byersdorfer, C.A. & Unanue, E.R. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J. Immunol. 183, 7909–7918 (2009).
Oh, J. et al. MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells. J. Exp. Med. 210, 1069–1077 (2013).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722 (2012).
Weiss, J.M. et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742 (2012).
Kurts, C. et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184, 923–930 (1996).
Olivares-Villagómez, D., Wang, Y. & Lafaille, J.J. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188, 1883–1894 (1998).
Cederbom, L., Hall, H. & Ivars, F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 30, 1538–1543 (2000).
Boyman, O., Kovar, M., Rubinstein, M.P., Surh, C.D. & Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311, 1924–1927 (2006).
Webster, K.E. et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 206, 751–760 (2009).
Pandiyan, P., Zheng, L., Ishihara, S., Reed, J. & Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8, 1353–1362 (2007).
de la Rosa, M., Rutz, S., Dorninger, H. & Scheffold, A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 34, 2480–2488 (2004).
Gillis, S. & Smith, K.A. Long term culture of tumour-specific cytotoxic T cells. Nature 268, 154–156 (1977).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12, 180–190 (2012).
Perry, J.S. et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41, 414–426 (2014).
Proietto, A.I. et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA 105, 19869–19874 (2008).
Malek, T.R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 (2002).
Román, E., Shino, H., Qin, F.X. & Liu, Y.J. Cutting edge: Hematopoietic-derived APCs select regulatory T cells in thymus. J. Immunol. 185, 3819–3823 (2010).
Aschenbrenner, K. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 (2007).
Hinterberger, M. et al. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat. Immunol. 11, 512–519 (2010).
Sansom, S.N. et al. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 24, 1918–1931 (2014).
Koble, C. & Kyewski, B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J. Exp. Med. 206, 1505–1513 (2009).
Busse, D. et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc. Natl. Acad. Sci. USA 107, 3058–3063 (2010).
Kim, H.P., Kelly, J. & Leonard, W.J. The basis for IL-2-induced IL-2 receptor α chain gene regulation: importance of two widely separated IL-2 response elements. Immunity 15, 159–172 (2001).
Barthlott, T. et al. CD25+CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int. Immunol. 17, 279–288 (2005).
Coquet, J.M. et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27–CD70 pathway. J. Exp. Med. 210, 715–728 (2013).
Mahmud, S.A. et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15, 473–481 (2014).
Mamalaki, C. et al. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA 89, 11342–11346 (1992).
Dzhagalov, I.L., Melichar, H.J., Ross, J.O., Herzmark, P. & Robey, E.A. in Current Protocols in Cytometry (ed., Robinson, J.P.) Ch 12, 12.26.1–12.26.20 (John Wiley and Sons, 2012).
Melichar, H.J., Ross, J.O., Herzmark, P., Hogquist, K.A. & Robey, E.A. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Sci. Signal. 6, ra92 (2013).
Ehrlich, L.I., Oh, D.Y., Weissman, I.L. & Lewis, R.S. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity 31, 986–998 (2009).
Halkias, J. et al. Opposing chemokine gradients control human thymocyte migration in situ. J. Clin. Invest. 123, 2131–2142 (2013).
Acknowledgements
We thank all the members of the Robey Lab for critical reading of the manuscript; H. Chu, H. Melichar and K. Taylor for technical assistance and suggestions; H. Nolla and A. Valeros for help with cell sorting; and N. Shastri (University of California, Berkeley) for the CTLL2 cell line. Supported by US National Institutes of Health (R01AI064227 to E.A.R. and T32AI100829 to B.M.W.).
Author information
Authors and Affiliations
Contributions
B.M.W. designed, performed and analyzed experiments and wrote the manuscript; N.K., J.B. and S.W.C. performed experiments; and E.A.R. supervised the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Time course of OT-II Treg cell development in thymic slices.
Time course of Treg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to thymic slices from mice that were previously injected with 2mg OVA protein. Data are representative of 4 independent experiments, gated on OT-II CD4SP.
Supplementary Figure 2 Treg cell development in thymic slices recapitulates normal thymic development rather than in vitro–induced Treg cell development.
(a) OT-II thymocytes or mature OT-II T cells were added to thymic slices from wild type mice that were injected with OVA protein 1hr prior to sacrifice. TGF-β (5ng/ml) was added along with mature OT-II T cells in some samples as indicated. (b) Neuropilin-1 expression levels on gated OT-II Foxp3+ CD4SP from thymocytes, splenocytes, or Foxp3 negative thymic CD4SP. (c) OT-II thymocytes were depleted of CD4SP prior to introduction into thymic slices. Flow cytometric analysis of samples before and after depletion are shown. (d) OT-II CD4SP depleted thymocytes overlaid onto WT thymic slices along with 1μM OVA peptide. Flow cytometry of gated OT-II CD4SP after days 3 or 4 of culture are shown. Data representative of 2 (a,b) or 3 (c,d) independent experiments.
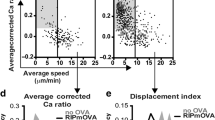
Supplementary Figure 3 Relationship between the frequency of precursors of Treg cells and Treg cell development.
The % Treg cells (CD25+Foxp3+) amongst OT-II donor thymocytes plotted versus the % of donor OT-II thymocytes within a thymic slice. Each dot represents the values for an individual thymic slice and data are compiled from 3-4 independent experiments. Ovalbumin was introduced via i.v. injection of mice prior to sacrifice and preparation of thymic slices (a), use of thymic slices from mice expressing a RIPmOVA transgene (b), or addition of OVA loaded bone marrow derived DCs to thymic slices containing OT-II thymocytes (c). Data compiled from multiple experiments, (a) n=15 (4 exp.); (b) n=16 (3 exp.); (c) n=19 (4 exp.).
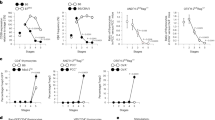
Supplementary Figure 4 Treg cell development is enhanced in the absence of existing thymic Treg cells.
(a) Slices from WT or AND (Treg deficient) thymi, overlaid with 2D2 TCR transgenic thymocytes in the presence of 3.8μM MOG 35-55 peptide. Treg cell development was analyzed on day 3 by flow cytometry. (b) Number of 2D2 Treg cells recovered per thymic slice after 3d. *p<0.01 by student’s t-test, n=6 and 3 slices respectively, representative of 2 independent experiments. (c) Slices from WT or F5 TCR transgenic Rag2-/-(Treg deficient) thymi, overlaid with OT-II thymocytes in the presence of the indicated concentrations of OVA peptide. Treg cell development analyzed on day 3 by flow cytometry. (d) Number of OT-II Treg cells recovered per thymic slice after 3d. *p<0.01 and **p<0.001 one-way ANOVA using Tukey’s post test analysis. Data pooled from 3 independent experiments, n=9 slices each.
Supplementary Figure 5 DCs derived from IL-2-deficient bone marrow have normal antigen-presentation capacity.
DCs were generated in culture from WT and Il2−/− bone marrow, incubated with 1mg/ml OVA protein, and introduced into thymic slices along with OT-II thymocytes. Flow cytometric analysis of activation markers on OT-II thymocytes was performed after 16h of culture. (a) Representative flow cytometry plots and (b) expression levels of activation markers on gated CD4SP OT-II thymocytes. Error bars represent +/- SEM, n=6 slices per condition, ns= not significant by unpaired student’s t-test, data representative of 3 independent experiments.
Supplementary Figure 6 Localized IL-2 production by the DC population presenting antigen.
Il2−/− thymic slices were overlaid with OT-II thymocytes followed by the addition of WT or Il2−/− DCs loaded with 1mg/ml OVA protein. OT-II Treg cell development was measured on d3. (a) Representative flow cytometry plots. (b) Quantification of the number of OT-II Treg cells recovered per thymic slice. *p<0.001 by student’s t-test. Data representative of 3 independent experiments, n=8 slices per condition. (c) Treg precursors (orange) interact with niche-defining APCs that bear agonist self-peptide (orange rectangles) and also supply IL2 (black dots). As precursors upregulate CD25 and differentiate into Treg cells (blue) they limit IL2 availability from the APC, providing negative feedback and preventing future Treg cell development within the niche. Shaded stars indicate cells in the surrounding tissue that are not part of the niche.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 734 kb)
Rights and permissions
About this article
Cite this article
Weist, B., Kurd, N., Boussier, J. et al. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol 16, 635–641 (2015). https://doi.org/10.1038/ni.3171
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3171
This article is cited by
-
Proinflammatory plasticity towards Th17 paradigm of regulatory T cells consistent with elevated prevalence of TGFBR2 variants in elderly patients with primary immune thrombocytopenia
BMC Immunology (2023)
-
Recirculating Foxp3+ regulatory T cells are restimulated in the thymus under Aire control
Cellular and Molecular Life Sciences (2022)
-
Factors that influence the thymic selection of CD8αα intraepithelial lymphocytes
Mucosal Immunology (2021)
-
Exploring the Pathogenic Role and Therapeutic Implications of Interleukin 2 in Autoimmune Hepatitis
Digestive Diseases and Sciences (2021)
-
The thymus medulla and its control of αβT cell development
Seminars in Immunopathology (2021)