Abstract
Follicular helper T cells (TFH cells) and follicular regulatory T cells (TFR cells) regulate the quantity and quality of humoral immunity. Although both cell types express the costimulatory receptor ICOS and require the transcription factor Bcl-6 for their differentiation, the ICOS-dependent pathways that coordinate their responses are not well understood. Here we report that activation of ICOS in CD4+ T cells promoted interaction of the p85α regulatory subunit of the signaling kinase PI(3)K and intracellular osteopontin (OPN-i), followed by translocation of OPN-i to the nucleus, its interaction with Bcl-6 and protection of Bcl-6 from ubiquitin-dependent proteasome degradation. Post-translational protection of Bcl-6 by OPN-i was essential for sustained responses of TFH cells and TFR cells and regulation of the germinal center B cell response to antigen. Thus, the p85α–OPN-i axis represents a molecular bridge that couples activation of ICOS to Bcl-6-dependent functional differentiation of TFH cells and TFR cells; this suggests new therapeutic avenues to manipulate the responses of these cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Johnston, R.J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Bunting, K.L. & Melnick, A.M. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr. Opin. Immunol. 25, 339–346 (2013).
Sage, P.T., Francisco, L.M., Carman, C.V. & Sharpe, A.H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2013).
Linterman, M.A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Baumjohann, D., Okada, T. & Ansel, K.M. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 187, 2089–2092 (2011).
Gigoux, M. et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 106, 20371–20376 (2009).
Xu, H. et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496, 523–527 (2013).
Kang, S.G. et al. MicroRNAs of the miR-17 approximately 92 family are critical regulators of TFH differentiation. Nat. Immunol. 14, 849–857 (2013).
Rolf, J. et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J. Immunol. 185, 4042–4052 (2010).
Yu, J. et al. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 18, 1379–1387 (1998).
Park, S.W. et al. The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16, 429–437 (2010).
Winnay, J.N., Boucher, J., Mori, M.A., Ueki, K. & Kahn, C.R. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat. Med. 16, 438–445 (2010).
Shinohara, M.L., Kim, H.J., Kim, J.H., Garcia, V.A. & Cantor, H. Alternative translation of Osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. USA 105, 7235–7239 (2008).
Cantor, H. & Shinohara, M.L. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat. Rev. Immunol. 9, 137–141 (2009).
Ashkar, S. et al. Eta-1 (osteopontin): an early component of type 1 (cell-mediated) immunity. Science 287, 860–864 (2000).
Shinohara, M.L. et al. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA 102, 17101–17106 (2005).
Shinohara, M.L. et al. Osteopontin expression is essential for IFN-α production by plasmacytoid dendritic cells. Nat. Immunol. 7, 498–506 (2006).
Patarca, R., Wei, F.Y., Iregui, M.V. & Cantor, H. Differential induction of interferon-gamma gene expression after activation of CD4+ T-cells by conventional antigen and Mls superantigen. Proc. Natl. Acad. Sci. USA 88, 2736–2739 (1991).
Shinohara, M.L., Kim, J.H., Garcia, V.A. & Cantor, H. Engagement of the type-I interferon receptor on dendritic cells inhibits promotion of Th17 cells: role of intracellular osteopontin. Immunity 29, 68–78 (2008).
Kerfoot, S.M. et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 34, 947–960 (2011).
Choi, Y.S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Chang, J.H. et al. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J. Exp. Med. 211, 137–151 (2014).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008).
Obenauer, J.C., Cantley, L.C. & Yaffe, M.B. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 31, 3635–3641 (2003).
Yaffe, M.B. et al. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 19, 348–353 (2001).
Choi, Y.S., Eto, D., Yang, J.A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013).
Nakayamada, S. et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J. Immunol. 192, 2156–2166 (2014).
Rolf, J., Fairfax, K. & Turner, M. Signaling pathways in T follicular helper cells. J. Immunol. 184, 6563–6568 (2010).
Xiao, N. et al. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol. 15, 657–666 (2014).
Inoue, M. & Shinohara, M.L. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 49, 160–172 (2011).
Vinuesa, C.G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Yu, D. et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007).
Glasmacher, E. et al. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat. Immunol. 11, 725–733 (2010).
Patarca, R., Wei, F.Y., Singh, P., Morasso, M.I. & Cantor, H. Dysregulated expression of the T-cell cytokine Eta-1 in CD4–8- lymphocytes during the development of murine autoimmune disease. J. Exp. Med. 172, 1177–1183 (1990).
Wong, C.K., Lit, L.C., Tam, L.S., Li, E.K. & Lam, C.W. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 602–606 (2005).
Crotty, S., Johnston, R.J. & Schoenberger, S.P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11, 114–120 (2010).
Powell, J.A. et al. Expression profiling of a hemopoietic cell survival transcriptome implicates osteopontin as a functional prognostic factor in AML. Blood 114, 4859–4870 (2009).
Tun, H.W. et al. Pathway analysis of primary central nervous system lymphoma. Blood 111, 3200–3210 (2008).
Cerchietti, L.C. et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat. Med. 15, 1369–1376 (2009).
Huang, C., Hatzi, K. & Melnick, A. Lineage-specific functions of Bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat. Immunol. 14, 380–388 (2013).
Polo, J.M. et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat. Med. 10, 1329–1335 (2004).
Krönke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Kim, H.J., Verbinnen, B., Tang, X., Lu, L. & Cantor, H. Inhibition of follicular T helper cells by CD8+ Treg is essential for self tolerance. Nature 467, 328–332 (2010).
Leavenworth, J.W., Tang, X., Kim, H.J., Wang, X. & Cantor, H. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J. Clin. Invest. 123, 1382–1389 (2013).
Leavenworth, J.W., Wang, X., Wenander, C.S., Spee, P. & Cantor, H. Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 108, 14584–14589 (2011).
Takahashi, K., Mitsui, K. & Yamanaka, S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature 423, 541–545 (2003).
Zhao, J.J. et al. The oncogenic properties of mutant p110α and p110β phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA 102, 18443–18448 (2005).
Lim, K.L. et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci. 25, 2002–2009 (2005).
Acknowledgements
We thank H. von Boehmer (Dana-Farber Cancer Institute) for B6.Foxp3GFP mice; L. Cantley (Weill Cornell Medical College) for p85α and Flag-p85α plasmids; T. Dawson (Johns Hopkins University) for the HA-Ub plasmid; S. Yamanaka (Kyoto University) for the HA-p110δ plasmid; J. Zhao (Dana-Farber Cancer Institute) for the HA-p110α plasmid; K. Wucherpfennig for critical reading; L. Cameron for help with image preparation and analysis; Y. Shao for microarray analysis; and A. Angel for preparation of the manuscript and figures. Supported by the US National Institutes of Health (AI48125 to H.C.; and T32 CA070083 to J.W.L.), The LeRoy Schecter Research Foundation (H.C.), the Benacerraf Society (J.W.L.) and the Belgian-American Educational Foundation (B.V.).
Author information
Authors and Affiliations
Contributions
J.W.L. and H.C. conceived of and planned experiments, analyzed data and wrote the paper; B.V. made the OPN-i-KI genomic construct; and J.W.L., J.Y. and H.H. performed experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
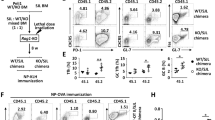
Supplementary Figure 1 Sorting and gating strategy.
Upper left, FACS plots show isolation of different CD4+ TH populations from B6 or OPN-i-KI mice after immunization with KLH in CFA. TN: CD4+CD44loCXCR5loPD-1loGITR− naïve cells; TFH: CD4+CD44hiCXCR5+PD-1+GITR− cells; TFR: CD4+CD44hiCXCR5+PD-1+GITR+ cells; Non-TFH: CD4+CD44hiCXCR5loPD-1loGITR− cells; Treg: CD4+CD44medCXCR5−PD-1−GITR+ cells. Bottom left, FACS plots show isolation of CD4+ TFH or TFR cells from OPN-i-KI or OPN-KO mice 5 d after immunization with KLH in CFA, shown in Fig. 2e. Gating control stains that lack (–) either anti-PD-1 or biotin-anti-CXCR5 using OPN-i-KI cells are shown. Upper right, gating strategy for TFH (CD4+CD44hiCXCR5+PD-1+Foxp3−), TFR (CD4+CD44hiCXCR5+PD-1+Foxp3+) and non-TFH (CD4+CD44hiCXCR5loPD-1lo) cells in Fig. 3a. Bottom right, Gating controls for defining the PD-1+CXCR5+ surface phenotype of CD4+ TFH and TFR cells in Fig. 3c. Negative control: CD4+CD44lo cells; Positive control (for CXCR5 stains): Fas+B220+ B cells; FMO PD-1 control: all antibodies except (–) anti-PD-1; CXCR5 control: all antibodies except (–) biotin-anti-CXCR5 (streptavidin-APC alone).
Supplementary Figure 2 Generation and confirmation of OPN-i-KI mice.
a, Spp1 genomic locus and targeting strategy. Boxes represent exons; exon 2 (gray) indicates the mutation site with deletion of the 45 nucleotides after the translational start site (ATG) that encode an N-terminal signal sequence while sparing other endogenous elements. A transcriptional STOP element flanked by loxP sites (black triangles) was inserted upstream of this mutation site to prevent OPN-i expression. Germline transmitted Spp1flstop/+ mice were backcrossed to B6 mice for at least 5 generations before crossing with mice carrying the Cre recombinase to allow OPN-i expression. neor, neomycin-resistance gene. b, PCR of genomic DNA showing wild-type, OPN-i-KI and OPN-KO mice after crossing with EIIa-Cre mice using genotyping primers indicated as gray triangles in a. OPN-KO mice gained the STOP element (194 bp) compared to wild-type allele. wild-type: 324 bp, OPN-i-KI (after Cre recombination): 453 bp, OPN-KO: 518 bp. c, Secreted OPN protein measured by ELISA from supernatants of purified DC, NK, T cells and peritoneal macrophages from each mouse strain after stimulation with the indicated reagents for 24 h or 2 d for macrophages. d, Immunoblot analysis of splenocyte lysates from the indicated mouse strains, probed with anti-OPN and anti-actin. Right, quantification of ratio of OPN to actin (n = 5 mice per group). e, Secreted IFN-α protein in pDC after stimulation by CpG-B (ODN-1668) (n = 3 mice per group) (***P < 0.001; error bars, mean ± s.e.m).
Supplementary Figure 3 OPN-i deficiency does not affect B cell activity or other helper T cell differentiation.
a, Quantification of CD44 expression (MFI) by CD4+ T cells, percent CD4+ T cells and Foxp3+CD44+CD4+ Treg cells from OT-II, OT-II OPN-KO and OT-II OPN-i-KI mice (as in Fig. 1b) 7 d post-challenge. Data represent at least three independent experiments with 6 mice per group (error bars, mean and ± s.e.m). b, Titer of total (NP23) and high-affinity (NP4) NP-specific IgG in the serum of Rag2−/− Prf1−/− hosts transferred with OT-II CD4+ T cells from OPN wild-type or OPN-KO mice and OPN wild-type or OPN-KO B cells followed by immunization with NP13-OVA in CFA and analysis 10 d later. Data represent two independent experiments with 4 mice per group. c, Frequency and numbers of donor CD45.2+Vβ5+CD4+ T cells and surface expression of CD44 by these cells from spleens of CD45.1 congenic recipients 7 d post-immunization with OVA in CFA. d, Flow cytometry of donor Vβ5+CD4+ T cells in c. Numbers adjacent to outlined areas indicate percent Bcl-6+CXCR5+ TFH cells and Vβ5+CD4+ T cells expressing intracellular cytokines. Below, frequency of TFH cells and cytokine-producing cells (n = 6 mice per group). *P < 0.05 (unpaired two-tailed Student’s t-test); NS, not significant. Error bars indicate mean ± s.e.m. e, Cytokine production by naïve CD44loCD25−CD4+ T cells purified from the indicated OT-II mice and differentiated for 5 d under TH1, TH2, TH17 and TFH conditions. *P < 0.05 (error bars, mean ± s.e.m of triplicate wells).
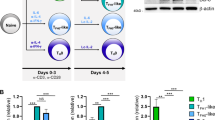
Supplementary Figure 4 Effects of OPN-i deficiency on Bcl-6 expression and the differentiation of inducible Treg cells and TFR cells.
a, Flow cytometry of CD25+Foxp3+ iTreg differentiated from sorted naïve CD25−CD4+ T cells, stimulated with plate-bound anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) in the presence of TGF-β1 (5 ng/ml) and hIL-2 (100 U/ml) for 5 d. b, iTregs from (a) were co-cultured with CFSE-labeled naïve CD25−CD4+ T cells (responder) activated with anti-CD3 and irradiated APC at different ratios. Histograms of CFSE dilutions, analyzed by flow cytometry, as readout of responder proliferation. Serum titers of total (NP23) and high-affinity (NP4) IgG (c) and anti-KLH (d) IgG from recipients in Fig. 2c (n = 5 mice per group). *P < 0.05, **P < 0.01 and ***P < 0.001 (unpaired two-tailed Student’s t-test; error bars, mean ± s.e.m). e, Immunoblot analysis of enriched CD44+CD4+ T cells from the indicated mice at days 1-15 after immunization with KLH in CFA, probed with the indicated Abs. Below, ratio of Bcl-6 to actin. f, RT-PCR analysis of Bcl6 and Prdm1 mRNA in CD44+CD4+ T cells purified from OPN-i-KI or OPN-KO mice from e. Bcl6 or Prdm1 expression was normalized to the Rps18 control and results are presented relative to that of OPN-i-KI mice at d1, set as 1. Data are representative of two independent experiments (e) or one experiment with 3 mice per time point (f; error bars, mean ± s.e.m).
Supplementary Figure 5 Microarray analysis of genes upregulated in CD4+ T cells by costimulation with ICOS.
a, Multiplot of genes upregulated in CD4+ T cells after restimulation with anti-CD3 and anti-ICOS (duplicates) compared to anti-CD3 alone (quadruplicates) as described in Fig. 4a. 210 (red) genes upregulated and 9 (blue) genes downregulated after co-ligation of CD3 and ICOS (cut-off 1.5 fold and P < 0.01). b, Functional analysis performed by Ingenuity pathway analysis (IPA) of 210 genes upregulated by ICOS co-stimulation in a. Functional annotations that are related to T-cell activation, differentiation, antibody production and antibody-mediated autoimmune disease with P values and numbers of genes are listed. c, Heatmap analysis displays 31 genes upregulated in ICOS-activated CD4+ T cells that correlate with systemic autoimmune syndrome revealed by IPA in b (P = 2.65 × 10-11).
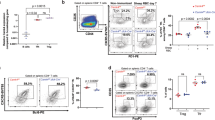
Supplementary Figure 6 OPN-i does not interact with p110, and p85α deficiency does not impair other helper T cell differentiation in vivo.
Immunoassay of lysates of 293T cells transfected with plasmids expressing HA-p110α (a) or HA-p110δ (b) and increasing concentrations of OPN-i, assessed by immunoprecipitation with anti-HA and immunoblot analysis with the indicated Abs. c, Purified CD44+CD4+ T cells from OPN-i-KI or OPN-KO mice 3 d after immunization with KLH and CFA were treated as in Fig. 4a. Quantification of ratios of phospho-Akt (pAkt) to total Akt by ELISA from cells after 30 min of crosslinking. d, Flow cytometry of splenocytes of OT-II OPN-i-KI or OT-II OPN-KO mice 3 d post-immunization with NP13-OVA in CFA, stimulated with (+) or without (–) IL-6 (20 ng/ml) for 15 min. Overlay of histograms of intracellular phospho-STAT1 and phospho-STAT3 among CD4+CD44+ T cells. e, Flow cytometry of splenocytes from p85α wild-type and p85α KO mice 3 d after injection with KLH and CFA. Numbers indicate percent Foxp3−Bcl-6+CXCR5+ TFH cells and Foxp3+Bcl-6+CXCR5+ TFR cells. Right, Bcl-6 MFI (n = 4 mice per group). **P < 0.01 (unpaired two-tailed Student’s t-test; error bars, mean ± s.e.m). Data represent two independent experiments. f, Quantification of numbers and surface CD44 expression of CD45.1−CD4+ donor cells from Fig. 5a. g, Gating controls for defining Bcl-6+CXCR5+ CD4+ TFH or TFR cells in Fig. 5a,b,d. CXCR5 control: all antibodies except (–) biotin-anti-CXCR5 (streptavidin-APC alone); Bcl-6 control: all antibodies except anti-Bcl-6; in this case, an IgG isotype-matched control for anti-Bcl-6 was used; Negative control: splenic CD44loCD4+ T cells from B6 mice at day 8 post-injection with KLH in CFA; Positive control: splenic CD44hiCD25medCD4+ T cells from B6 mice at d8 post-immunization with KLH in CFA; or Bcl-6+CXCR5+ cells in CD19+ B cells from Tcrα−/– recipients of p85α KO Treg in Fig. 5b. h, Flow cytometry of donor CD45.1−CD4+ T cells from Fig. 5a, stimulated with PMA and Ionomycin for 5 h. Numbers indicate percent CD4+ T cells expressing intracellular cytokines. Right, frequency of cytokine-producing CD4+ T cells. Data represent two independent experiments with 3-4 mice per group (error bars, mean ± s.e.m). i, Immunoassay of lysates of 293T cells transfected with vectors expressing Flag-p85α and OPN-i, treated with calf intestinal phosphatase (CIP), and assessed by immunoprecipitation with anti-Flag followed by immunoblot analysis. j, Diagram of a short sequence motif of OPN with a tyrosine at position 166 that may interact with the p85α SH2 domain. k, Expression of surface ICOS receptor and intracellular Bcl-6 in CD44+CD4+ T cells from OPN-i-KI mice 3 d after immunization with KLH in CFA.
Supplementary Figure 7 Wild-type OPN-i interacts with and stabilizes Bcl-6, but the Y166F OPN-i mutant does not.
a, Confocal microscopy of 293T cells transfected with plasmids encoding p85α, Flag–Bcl-6 and OPN-i–GFP wild-type or OPN-i–GFP Y166F mutant, assessed by pre-extraction of soluble nuclear proteins with 0.5% Triton X-100 after 24 h of transfection followed by immunostaining as indicated. Yellow in the merged image shows colocalization of Bcl-6 and OPN-i wild-type. b, Immunoassay of nuclear and cytosolic fractions of 293T cells transfected with plasmids encoding Flag–Bcl-6, OPN-i wild-type or OPN-i Y166F mutant, assessed by immunoprecipitation (IP) with anti-Flag and then immunoblot analysis. c, Top, Illustration of Bcl-6 protein deletion mutants. Immunoassay of lysates of 293T cells transfected with plasmids encoding OPN-i and Flag–Bcl-6 wild-type or deletion mutants, assessed by IP and immunoblot analysis as in b. Right, immunoassay of lysates of 293T cells transfected with plasmids encoding OPN-i–Flag and Bcl-6 ZF deletion mutant (no Flag tag) followed by IP with anti-Flag and immunoblot with anti-Bcl-6 and anti-Flag. Arrowhead: IgG heavy chain. d, Immunoblot analysis of lysates of 293T cells transfected with plasmids encoding OPN-i (100 ng) and graded concentrations of Flag–Bcl-6 wild-type or deletion mutants (lane 1,4,7,10: 450 ng; 2,5,8,11: 300 ng; 3,6,9,12: 150 ng). Cell lysates from lanes 1,4,7,10 were used for immunoassay in Fig. 7b. e, Immunoblot analysis of lysates of 293T cells transfected with plasmids encoding Flag–Bcl-6 and/or OPN-i, treated with (+) or without (–) DUBi for 8 h, probed with anti-Flag and anti-actin. Below lanes, ratio of Flag (Bcl-6) to actin.
Supplementary Figure 8 Schematic of sustenance of Bcl-6-dependent follicular T cell differentiation mediated by the p85α–OPN-i axis.
Engagement of ICOS and TCR on CD4+ T cells by APC (e.g., DC) promotes p85α–OPN-i complex formation that requires the tyrosine site 166 of OPN-i. p85α chaperones OPN-i entry into the nucleus, where intranuclear OPN-i interacts with Bcl-6 via the sequences within the RD2 and protects Bcl-6 from ubiquitination-mediated degradation. This p85α–OPN-i axis connects ICOS signals to stable Bcl-6 expression (highlighted in blue) and ensures functional follicular T cell differentiation program.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 842 kb)
Rights and permissions
About this article
Cite this article
Leavenworth, J., Verbinnen, B., Yin, J. et al. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol 16, 96–106 (2015). https://doi.org/10.1038/ni.3050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3050
This article is cited by
-
The TRIM37 variants in Mulibrey nanism patients paralyze follicular helper T cell differentiation
Cell Discovery (2023)
-
Osteopontin in autoimmune disorders: current knowledge and future perspective
Inflammopharmacology (2022)
-
Differentiation, functions, and roles of T follicular regulatory cells in autoimmune diseases
Inflammation and Regeneration (2021)
-
Dysregulated follicular regulatory T cells and antibody responses exacerbate experimental autoimmune encephalomyelitis
Journal of Neuroinflammation (2021)
-
Remodeling of the tumor microenvironment via disrupting Blimp1+ effector Treg activity augments response to anti-PD-1 blockade
Molecular Cancer (2021)