Abstract
Excessive activation of dendritic cells (DCs) leads to the development of autoimmune and inflammatory diseases, which has prompted a search for regulators of DC activation. Here we report that Rhbdd3, a member of the rhomboid family of proteases, suppressed the activation of DCs and production of interleukin 6 (IL-6) triggered by Toll-like receptors (TLRs). Rhbdd3-deficient mice spontaneously developed autoimmune diseases characterized by an increased abundance of the TH17 subset of helper T cells and decreased number of regulatory T cells due to the increase in IL-6 from DCs. Rhbdd3 directly bound to Lys27 (K27)-linked polyubiquitin chains on Lys302 of the modulator NEMO (IKKγ) via the ubiquitin-binding–association (UBA) domain in endosomes. Rhbdd3 further recruited the deubiquitinase A20 via K27-linked polyubiquitin chains on Lys268 to inhibit K63-linked polyubiquitination of NEMO and thus suppressed activation of the transcription factor NF-κB in DCs. Our data identify Rhbdd3 as a critical regulator of DC activation and indicate K27-linked polyubiquitination is a potent ubiquitin-linked pattern involved in the control of autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Accessions
Gene Expression Omnibus
NCBI Reference Sequence
References
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Miossec, P., Korn, T. & Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361, 888–898 (2009).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Littman, D.R. & Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Kimura, A. & Kishimoto, T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 40, 1830–1835 (2010).
O'Neill, L.A., Golenbock, D. & Bowie, A.G. The history of Toll-like receptors—redefining innate immunity. Nat. Rev. Immunol. 13, 453–460 (2013).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
DiDonato, J.A., Mercurio, F. & Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 (2012).
Ganguly, D., Haak, S., Sisirak, V. & Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 13, 566–577 (2013).
Boone, D.L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Nakagawa, R. et al. SOCS-1 participates in negative regulation of LPS responses. Immunity 17, 677–687 (2002).
Urban, S. & Dickey, S.W. The rhomboid protease family: a decade of progress on function and mechanism. Genome Biol. 12, 231 (2011).
Lemberg, M.K. & Freeman, M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Res. 17, 1634–1646 (2007).
Adrain, C., Zettl, M., Christova, Y., Taylor, N. & Freeman, M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science 335, 225–228 (2012).
McIlwain, D.R. et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science 335, 229–232 (2012).
Ding, S. et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483 (2005).
Xu, R. et al. A large-scale functional approach to uncover human genes and pathways in Drosophila. Cell Res. 18, 1114–1127 (2008).
Bahar, A. et al. Isolation and characterization of a novel pituitary tumor apoptosis gene. Mol. Endocrinol. 18, 1827–1839 (2004).
Bhoj, V.G. & Chen, Z.J. Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 (2009).
Chen, Z.J. Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246, 95–106 (2012).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Tokunaga, F. et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat. Cell Biol. 11, 123–132 (2009).
Xia, Z.P. et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 (2009).
Sebban-Benin, H. et al. Identification of TRAF6-dependent NEMO polyubiquitination sites through analysis of a new NEMO mutation causing incontinentia pigmenti. Hum. Mol. Genet. 16, 2805–2815 (2007).
Zhou, H. et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 (2004).
Abbott, D.W. et al. Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol. Cell. Biol. 27, 6012–6025 (2007).
Ma, A. & Malynn, B.A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 12, 774–785 (2012).
Hennessy, E.J., Parker, A.E. & O'Neill, L.A. Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307 (2010).
Ashida, H. et al. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKγ to dampen the host NF-κB-mediated inflammatory response. Nat. Cell Biol. 12, 66–73 (2010).
Arimoto, K. et al. Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc. Natl. Acad. Sci. USA 107, 15856–15861 (2010).
Zanoni, I. et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 (2011).
Donnelly, S. et al. Helminth cysteine proteases inhibit TRIF-dependent activation of macrophages via degradation of TLR3. J. Biol. Chem. 285, 3383–3392 (2010).
Liao, W. et al. CARP-2 is an endosome-associated ubiquitin protein ligase for RIP and regulates TNF-induced NF-κB activation. Curr. Biol. 18, 641–649 (2008).
Miaczynska, M., Pelkmans, L. & Zerial, M. Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16, 400–406 (2004).
Lin, A.E. et al. ARIH2 is essential for embryogenesis, and its hematopoietic deficiency causes lethal activation of the immune system. Nat. Immunol. 14, 27–33 (2013).
Wald, D. et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 4, 920–927 (2003).
Liu, J. et al. Rhomboid domain-containing protein 3 is a negative regulator of TLR3-triggered natural killer cell activation. Proc. Natl. Acad. Sci. USA 110, 7814–7819 (2013).
Hammer, G.E. et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 12, 1184–1193 (2011).
Kool, M. et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35, 82–96 (2011).
Lee, E.G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Yarilina, A., Park-Min, K.H., Antoniv, T., Hu, X. & Ivashkiv, L.B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9, 378–387 (2008).
Zahn, A. et al. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J. Gastroenterol. 13, 1687–1695 (2007).
Vinuesa, C.G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Xia, M. et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity 39, 470–481 (2013).
Xu, S. et al. Constitutive MHC class I molecules negatively regulate TLR -triggered inflammatory responses via the Fps-SHP-2 pathway. Nat. Immunol. 13, 551–559 (2012).
Chen, W. et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell 152, 467–478 (2013).
Han, C. et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 11, 734–742 (2010).
Wirtz, S., Neufert, C., Weigmann, B. & Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541–546 (2007).
Acknowledgements
We thank J. Jiang for technical assistance; C. Dong (MD Anderson Cancer Center) for Il17−/− mice; S. Jung (Weizmann Institute of Science) for CD11c-DTR mice; and J. Jung (Harvard Medical School) for the pEBG plasmid. Supported by the National Key Basic Research Program of China (2013CB530503 and 2012CB910202), the National Natural Science Foundation of China (81230074, 81123006 and 81172787) and the National 125 Key Project (2012AA020901).
Author information
Authors and Affiliations
Contributions
X.C. designed and supervised the research; J.L., C.H., B.X., Y.W., S.L., K.C., M.X., Y. Zhang, L.S., Z.L., T.Z. and F.M. did experiments; Q.W., J.W., K.D., Y. Zhuang, X.W., Y.Y. and T.X. contributed reagents and analytical tools; J.L., C.H., B.X., Y.W., S.L., M.X. and X.C. analyzed data; and J.L., C.H. and X.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
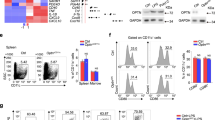
Supplementary Figure 1 Enhanced proliferation and Th1/Th17 differentiation of Rhbdd3–/– CD4+ T cells.
(a) Appearances of spleens and inguinal lymph nodes (LNs) from 6-month-old Rhbdd3–/– and Rhbdd3+/+ mice (n=3 mice per group). (b) Rhbdd3 mRNA expression in each of the indicated organs and immune cell types were measured by RT-PCR. (c, d) The percentages of CD4+CD25+Foxp3+ Treg in the spleen of Rhbdd3–/– and Rhbdd3+/+ mice were identified by FACS (c) and quantified (d, n=3 mice per group). The dot plots were gated on CD4+ T cells. (e) Proliferation of splenic CD4+ T cells stimulated with α-CD3/28. (f) Production of IFN-γ and IL-17 by MLN (mesenteric LNs) cells, splenocytes or CD4+ T cells stimulated with α-CD3/28. (g, h) Percentages of Th1 and Th17 cells in splenic T cells after α-CD3/CD28 stimulation were analyzed by FACS (g) and quantified (h). Med, Medium. *P<0.05, **P<0.01, ns, not significant (two tailed Student's t-test). Data are from three independent experiments (mean±s.d. of three mice (d) or of technical triplicates (f, h)) or are representative of three independent experiments (a-c, e, g).
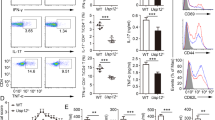
Supplementary Figure 2 More severe colitis and overactivation of immune cells in Rhbdd3–/– mice upon TNBS induction.
(a-d) TNBS-colitis was induced in 8-week-old Il17+/+ and Il17–/– mice (n=5 mice per group). Production of IL-17 in colon tissue was measured by CBA test (a). Percentages of weight changes were monitored daily (b). Appearances and histological sections of colons were examined at day3 after TNBS-induction (c, d). (e, f) TNBS-colitis was induced in 8-week-old Rhbdd3+/+ and Rhbdd3–/– mice (n=5 per group). Reduction of colon length was examined at day 3 after TNBS induction (e). Percentages of CD40, CD80, CD86 and I-Ab positive CD11c+ cells and CD44 positive CD4+ T cells in splenocytes were determined at day 3 after TNBS induction (f). *P<0.05, **P<0.01 (two tailed Student's t-test). Data are from three independent experiments (mean±s.d. of five mice (a, b, c (right), e (right), f) or are representative of three independent experiments with similar results (c (left), d, e (left)).
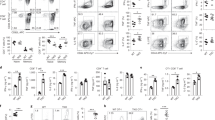
Supplementary Figure 3 Rhbdd3 promotes the generation of Treg and inhibits the differentiation of Th1 and Th17 by inhibiting IL-6 production from DC.
(a-c) CFSE-labeled OT-II CD4+ T cells were co-cultured with TLR-activated BMDCs (a) or splenic DCs (b, c) which were pretreated with OVA323-339 peptide at a ratio of 10:1. After 4 days of culture, proliferation rate of T cells were determined by CFSE dilution (a, b) and secretion of IFN-γ and IL-17 in co-culture supernatants were measured by CBA kit (c). (d) Percentages of CD4+CD25+ and CD4+Foxp3+ Treg in the spleens of Rhbdd3+/+, Rhbdd3–/–, Rhbdd3–/–Il6–/– or Il6–/– mice were measured by FACS (n=3 mice per group). (e) Production of IFN-γ and IL-17 by CD4+ T cells from Rhbdd3+/+, Rhbdd3–/–, Rhbdd3–/–Il6–/– or Il6–/– mice stimulated with α-CD3/CD28 were determined by CBA test. (f, g) CD11c-DTR mice depleted of DC were administrated with Rhbdd3+/+ or Rhbdd3–/– BMDCs. After 3 days, TNBS-induced colitis was performed in recipient mice. Weight loss were monitored daily (f, n=3 mice per group) and histology of colon sections were examined by H&E staining at day3 after TNBS induction (g). *P<0.05, **P<0.01 (two tailed Student's t-test). Data are from three independent experiments (mean±s.d. of three mice (d, f) or of technical triplicates (a, c, e) or are representative of three independent experiments with similar results (b, g).
Supplementary Figure 4 Rhbdd3 controls T cell homeostasis through inhibiting IL-6 production while does not affect T cell-intrinsic signaling activation.
(a) CD4+ T cells from Rhbdd3+/+ and Rhbdd3–/– mice were cultured under distinct T cell subset polarization conditions and analyzed for Th1, Th2, Th17, iTreg cell differentiation. (b, c) CD4+ T cells (b) and CD4+CD44lowCD62Lhigh naïve T cells (c) were stimulated with α-CD3/28 and assessed for MAPK and NF-κB signaling activation. Data are from one representative of three independent experiments with similar results.
Supplementary Figure 5 Rhbdd3 negatively regulates TLR and TNF-α-induced NF-κB pathways in BMDCs but not in BMDMs and MEF.
(a) Immunoblot of NF-κB and MAPK signaling proteins in Rhbdd3+/+ and Rhbdd3–/– BMDCs stimulated with CpG, Poly(I:C) and TNF. (b) EMSA analysis of NF-κB DNA binding ability in nuclear extracts of Rhbdd3+/+ and Rhbdd3–/– BMDCs stimulated with LPS. (c, d) Immunoblot of NF-κB and/or MAPK signaling proteins in Rhbdd3+/+ and Rhbdd3–/– BMDCs and BMDMs (c) and MEFs (d) stimulated with LPS. Data are from one representative of three independent experiments with similar results.
Supplementary Figure 6 Rhbdd3 interacts with K27-linked polyubiquitin chains on NEMO.
(a) Immunoblot in HEK293 cell lysates after single and sequential V5 IP. (b) Immunoblot in HEK293 cell lysates after sequential V5 IP as in (a). Numbers below HA-lanes indicate densitometry of HA relative to V5 expression in that same lane. (c) Immunoblot in HEK293 cell lysates after Flag IP. (d) Immunoblot in HEK293 cell lysates after V5 IP. (e) Silver staining of in vitro purified GST proteins. (f) Schematic of IP procedure in Fig. 6f. (g) Immunoblot in HEK293 cell lysates after V5 IP. Data are from one representative of three independent experiments with similar results.
Supplementary Figure 7 K27-linked polyubiquitination mediated the interaction among Rhbdd3, A20 and NEMO.
(a) Immunoblot of cell lysates immunoprecipitated by single or sequential V5 IP in HEK293 cells overexpressed with V5-tagged Rhbdd3 and HA-tagged one-K ubiquitin mutants. In single V5 IP, cell lysates were immunoprecipitated with anti-V5; in sequential V5 IP, proteins immunoprecipitated by anti-V5 were boiled in presence of 1% SDS and subjected to a second IP by anti-V5. (b) Immunoblot of cell lysates immunoprecipitated by Myc IP in HEK293 cells overexpressed with Flag-tagged Rhbdd3 and Myc-tagged A20. (c) Schematic of IP procedure in Fig. 7f. (d) Immunoblot of cell lysates immunoprecipitated by Myc IP followed by HA IP in HEK293 cells transfected as indicated. Data are from one representative of three independent experiments with similar results.
Supplementary Figure 8 Proposed working model for negative regulation of TLR-triggered NEMO/NF-κB activation and control of autoimmunity by Rhbdd3.
(a) (I) Activation of NEMO/NF-κB pathway upon TLR stimulation. Stimulation of TLR agonists activates IRAK1 and TRAF6. TRAF6 acts as an E3 ubiquitin ligase to activate the TAK1 complex, inducing IKK complex consisting of IKKα, IKKβ and NEMO. Activated IKK complex then phosphorylates IκB and leads to its dissociation from NF-κB. Freed NF-κB translocates into the nucleus and eventually initiates production of proinflammatory cytokines such as IL-6. (II) Inhibition of TLR-triggered NEMO/NF-κB activation by Rhbdd3. TLR signal promotes aggregation of Rhbdd3 in endosomes. Rhbdd3 directly binds to K27-linked polyubiquitin chains on K302 of NEMO via UBA domain. Rhbdd3 also recruits and interacts with A20 via K27-linked polyubiquitin chains on K268 and facilitates A20-mediated K63-linked deubiquitination on NEMO. Above signals lead to inhibition of IκBα degradation and NF-κB activation, and eventually inhibit the production of proinflammatory cytokines such as IL-6. (b) Activation of DC via TLRs leads to activation of NF-κB and production of IL-6, which then potently promotes Th17 generation while inhibits Treg generation, contributing to the development of autoimmune diseases. Rhbdd3 accumulates in endosome upon TLR stimulation and selectively inhibits TLR-triggered NF-κB activation and IL-6 production in DC by interacting with A20 and NEMO and facilitating K63-linked deubiquitination of NEMO. The inhibition of IL-6 production from DC by Rhbdd3 leads to the suppression of Th17 generation but promotion of Treg generation, and eventually contributing to the control of autoimmunity and prevention of autoimmune diseases. Abbreviations: IκBα, Inhibitor of NF-κB alpha; IKKα/β, IκB kinase α/β IRAK1, Interleukin-1 receptor-associated kinase 1; K27/K63, K27/K63-linked polyubiquitin chains; TAB1, TAK-binding protein 1; TAB2/3, TAK-binding protein 2/3 ; TAK1, Transforming growth factor β-activated kinase 1; TRAF6, TNF receptor-associated factor 6; NEMO, NF-κB essential modulator; NF-κB, Nuclear factor kappa B; UBA, Ubiquitin-binding associated domain.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 1497 kb)
Supplementary Table 1
Primer sequence used for RT-PCR and quantitative PCR. (PDF 69 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Han, C., Xie, B. et al. Rhbdd3 controls autoimmunity by suppressing the production of IL-6 by dendritic cells via K27-linked ubiquitination of the regulator NEMO. Nat Immunol 15, 612–622 (2014). https://doi.org/10.1038/ni.2898
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2898
This article is cited by
-
EFHD2 suppresses intestinal inflammation by blocking intestinal epithelial cell TNFR1 internalization and cell death
Nature Communications (2024)
-
N6-methyladenosine in myeloid cells: a novel regulatory factor for inflammation-related diseases
Journal of Physiology and Biochemistry (2023)
-
Immune-regulating strategy against rheumatoid arthritis by inducing tolerogenic dendritic cells with modified zinc peroxide nanoparticles
Journal of Nanobiotechnology (2022)
-
Whole-genome sequence analysis reveals selection signatures for important economic traits in Xiang pigs
Scientific Reports (2022)
-
RHBDF2 gene functions are correlated to facilitated renal clear cell carcinoma progression
Cancer Cell International (2021)