Abstract
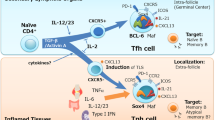
Antibody responses are classified according to whether B cells receive help from T cells—that is, whether they are thymus-dependent (TD) responses or thymus-independent (TI) responses. The latter can be elicited by microbial ligands (TI type 1) or by extensive crosslinking of the B cell antigen receptor (BCR; TI type 2). The hallmark of a TD response is the induction of germinal centers in which follicular helper T cells (TFH cells) select B cells with somatically mutated high-affinity BCRs to become memory cells. Studies have shown that B cells can also receive innate TD help from natural killer T cells (NKT cells) and innate TI help from cells such as neutrophils but that the outcome of such help differs from conventional TD and TI responses. Here we update the classification of antibody responses to take into account these emerging types of B cell helpers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
MacLennan, I.C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Tarlinton, D.M. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol. Cell Biol. 86, 133–138 (2008).
Gershon, R.K. & Kondo, K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18, 723–737 (1970).
Bretscher, P. & Cohn, M. A theory of self-nonself discrimination. Science 169, 1042–1049 (1970).
Noelle, R.J. & Snow, E.C. Cognate interactions between helper T cells and B cells. Immunol. Today 11, 361–368 (1990).
Cyster, J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 11, 989–996 (2010).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Vinuesa, C.G., Tangye, S.G., Moser, B. & Mackay, C.R. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5, 853–865 (2005).
Cannons, J.L. et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 32, 253–265 (2010).
Smith, K.G., Hewitson, T.D., Nossal, G.J. & Tarlinton, D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26, 444–448 (1996).
MacLennan, I.C. et al. Extrafollicular antibody responses. Immunol. Rev. 194, 8–18 (2003).
Vinuesa, C.G. & Cyster, J.G. How T cells earn the follicular rite of passage. Immunity 35, 671–680 (2011).
Victora, G.D. et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143, 592–605 (2010).
Liu, Y.J. et al. Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931 (1989).
Good-Jacobson, K.L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11, 535–542 (2010).
Bryant, V.L. et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179, 8180–8190 (2007).
Linterman, M.A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Winter, O. et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood 116, 1867–1875 (2010).
Mosier, D.E., Mond, J.J. & Goldings, E.A. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J. Immunol. 119, 1874–1878 (1977).
Mond, J.J., Lees, A. & Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692 (1995).
Bekeredjian-Ding, I. & Jego, G. Toll-like receptors–sentries in the B-cell response. Immunology 128, 311–323 (2009).
Peng, S.L. Signaling in B cells via Toll-like receptors. Curr. Opin. Immunol. 17, 230–236 (2005).
Alugupalli, K.R., Akira, S., Lien, E. & Leong, J.M. MyD88- and Bruton's tyrosine kinase-mediated signals are essential for T cell-independent pathogen-specific IgM responses. J. Immunol. 178, 3740–3749 (2007).
Eckl-Dorna, J. & Batista, F.D. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood 113, 3969–3977 (2009).
Minguet, S. et al. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur. J. Immunol. 38, 2475–2487 (2008).
Lanzavecchia, A. & Sallusto, F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr. Opin. Immunol. 19, 268–274 (2007).
Martin, F., Oliver, A.M. & Kearney, J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14, 617–629 (2001).
Braley-Mullen, H. Antigen requirements for induction of B-memory cells: studies with dinitrophenyl coupled to T-dependent and T-independent carriers. J. Exp. Med. 147, 1824–1831 (1978).
Garcia de Vinuesa, C., O'Leary, P., Sze, D.M., Toellner, K.M. & MacLennan, I.C. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 29, 1314–1323 (1999).
Garcia de Vinuesa, C. et al. Germinal centers without T cells. J. Exp. Med. 191, 485–494 (2000).
Lentz, V.M. & Manser, T. Cutting edge: germinal centers can be induced in the absence of T cells. J. Immunol. 167, 15–20 (2001).
Weller, S. et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104, 3647–3654 (2004).
Allman, D. & Pillai, S. Peripheral B cell subsets. Curr. Opin. Immunol. 20, 149–157 (2008).
Genestier, L. et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J. Immunol. 178, 7779–7786 (2007).
Cariappa, A. et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity 23, 397–407 (2005).
Martin, F. & Kearney, J.F. Marginal-zone B cells. Nat. Rev. Immunol. 2, 323–335 (2002).
Liu, Y.J., Oldfield, S. & MacLennan, I.C. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur. J. Immunol. 18, 355–362 (1988).
Zandvoort, A. & Timens, W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin. Exp. Immunol. 130, 4–11 (2002).
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J.V. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1, 31–36 (2000).
Song, H. & Cerny, J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 198, 1923–1935 (2003).
Phan, T.G., Gardam, S., Basten, A. & Brink, R. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. J. Immunol. 174, 4567–4578 (2005).
Cinamon, G., Zachariah, M.A., Lam, O.M., Foss, F.W. Jr. & Cyster, J.G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 9, 54–62 (2008).
Zietara, N., Lyszkiewicz, M., Krueger, A. & Weiss, S. ICOS-dependent stimulation of NKT cells by marginal zone B cells. Eur. J. Immunol. 41, 3125–3134 (2011).
Hayakawa, K., Hardy, R.R. & Herzenberg, L.A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161, 1554–1568 (1985).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293–301 (2006).
Alugupalli, K.R. et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390 (2004).
Defrance, T., Taillardet, M. & Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 23, 330–336 (2011).
Baumgarth, N., Tung, J.W. & Herzenberg, L.A. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26, 347–362 (2005).
Fagarasan, S. & Honjo, T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 3, 63–72 (2003).
Macpherson, A.J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Hardy, R.R. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr. Opin. Immunol. 18, 547–555 (2006).
Barral, P. et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. USA 105, 8345–8350 (2008).
Chang, P.P. et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43 (2012).
Detre, C. et al. SAP expression in invariant NKT cells is required for cognate help to support B-cell responses. Blood 120, 122–129 (2012).
Leadbetter, E.A. et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. USA 105, 8339–8344 (2008).
Bendelac, A., Savage, P.B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Venkataswamy, M.M. & Porcelli, S.A. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin. Immunol. 22, 68–78 (2010).
Godfrey, D.I. & Berzins, S.P. Control points in NKT-cell development. Nat. Rev. Immunol. 7, 505–518 (2007).
Kitamura, H. et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1128 (1999).
King, I.L. et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 13, 44–50 (2012).
Tonti, E. et al. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4+ T cell help. J. Immunol. 188, 3217–3222 (2012).
Fujii, S., Shimizu, K., Hemmi, H. & Steinman, R.M. Innate Vα14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 220, 183–198 (2007).
Puga, I. et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13, 170–180 (2012).
Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Mackay, F. & Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 (2009).
Mosier, D.E. & Subbarao, B. Thymus-independent antigens: complexity of B-lymphocyte activation revealed. Immunol. Today 3, 217–222 (1982).
Garcia De Vinuesa, C. et al. Dendritic cells associated with plasmablast survival. Eur. J. Immunol. 29, 3712–3721 (1999).
Balazs, M., Martin, F., Zhou, T. & Kearney, J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352 (2002).
Randolph, G.J., Jakubzick, C. & Qu, C. Antigen presentation by monocytes and monocyte-derived cells. Curr. Opin. Immunol. 20, 52–60 (2008).
Leon, B. & Ardavin, C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol. Cell Biol. 86, 320–324 (2008).
Tezuka, H. et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 (2007).
Craxton, A., Magaletti, D., Ryan, E.J. & Clark, E.A. Macrophage- and dendritic cell–dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101, 4464–4471 (2003).
Merluzzi, S. et al. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood 115, 2810–2817 (2010).
Gauchat, J.F. et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature 365, 340–343 (1993).
Erazo, A. et al. Unique maturation program of the IgE response in vivo. Immunity 26, 191–203 (2007).
Shinkai, K., Mohrs, M. & Locksley, R.M. Helper T cells regulate type-2 innate immunity in vivo. Nature 420, 825–829 (2002).
Wang, H.B. & Weller, P.F. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J. Leukoc. Biol. 83, 817–821 (2008).
Henz, B.M., Maurer, M., Lippert, U., Worm, M. & Babina, M. Mast cells as initiators of immunity and host defense. Exp. Dermatol. 10, 1–10 (2001).
Sokol, C.L. et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 10, 713–720 (2009).
Spencer, L.A. & Weller, P.F. Eosinophils and Th2 immunity: contemporary insights. Immunol. Cell Biol. 88, 250–256 (2010).
Tacke, F. et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J. Exp. Med. 203, 583–597 (2006).
Chorny, A., Puga, I. & Cerutti, A. Innate signaling networks in mucosal IgA class switching. Adv. Immunol. 107, 31–69 (2010).
Chu, V.T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159 (2011).
Mack, M. et al. Identification of antigen-capturing cells as basophils. J. Immunol. 174, 735–741 (2005).
Kim, S., Shen, T. & Min, B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J. Immunol. 183, 3033–3039 (2009).
Denzel, A. et al. Basophils enhance immunological memory responses. Nat. Immunol. 9, 733–742 (2008).
Ruprecht, C.R. & Lanzavecchia, A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 36, 810–816 (2006).
Gavin, A.L. et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314, 1936–1938 (2006).
Pasare, C. & Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 438, 364–368 (2005).
Day, N. et al. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J. Pediatr. 144, 524–526 (2004).
Ku, C.L. et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J. Med. Genet. 44, 16–23 (2007).
Sweet, R.A. et al. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proc. Natl. Acad. Sci. USA 108, 7932–7937 (2011).
Snapper, C.M. et al. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 22, 308–311 (2001).
Khan, A.Q., Chen, Q., Wu, Z.Q., Paton, J.C. & Snapper, C.M. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect. Immun. 73, 298–307 (2005).
Chu, V.T. & Berek, C. Immunization induces activation of bone marrow eosinophils required for plasma cell survival. Eur. J. Immunol. 42, 130–137 (2012).
Wollenberg, I. et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187, 4553–4560 (2011).
Linterman, M.A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Chan, T.D. et al. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 183, 3139–3149 (2009).
Inamine, A. et al. Two waves of memory B-cell generation in the primary immune response. Int. Immunol. 17, 581–589 (2005).
Toyama, H. et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity 17, 329–339 (2002).
Hsu, M.C., Toellner, K.M., Vinuesa, C.G. & Maclennan, I.C. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc. Natl. Acad. Sci. USA 103, 5905–5910 (2006).
Obukhanych, T.V. & Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203, 305–310 (2006).
Nie, Y. et al. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J. Exp. Med. 200, 1145–1156 (2004).
Taillardet, M. et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood 114, 4432–4440 (2009).
Gaspal, F.M. et al. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. Eur. J. Immunol. 36, 1665–1673 (2006).
Toellner, K.M. et al. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J. Exp. Med. 195, 383–389 (2002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vinuesa, C., Chang, PP. Innate B cell helpers reveal novel types of antibody responses. Nat Immunol 14, 119–126 (2013). https://doi.org/10.1038/ni.2511
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2511
This article is cited by
-
Enhancing antibody responses by multivalent antigen display on thymus-independent DNA origami scaffolds
Nature Communications (2024)
-
Type I Interferon Potentiates IgA Immunity to Respiratory Syncytial Virus Infection During Infancy
Scientific Reports (2018)
-
Regulation of age-associated B cells by IRF5 in systemic autoimmunity
Nature Immunology (2018)
-
A generalized quantitative antibody homeostasis model: regulation of B‐cell development by BCR saturation and novel insights into bone marrow function
Clinical & Translational Immunology (2017)
-
A generalized quantitative antibody homeostasis model: antigen saturation, natural antibodies and a quantitative antibody network
Clinical & Translational Immunology (2017)