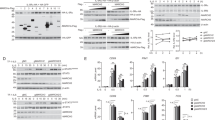
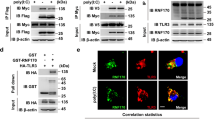
Abstract
The ST2L receptor for interleukin 33 (IL-33) mediates pulmonary inflammation and immune system–related disorders, such as asthma and rheumatoid arthritis. At present, very little is known about the molecular regulation of ST2L expression. Here we found that FBXL19, an 'orphan' member of the Skp1–Cullin–F-box family of E3 ubiquitin ligases, selectively bound to ST2L to mediate its polyubiquitination and elimination in the proteasome. Degradation of ST2L involved phosphorylation of ST2L at Ser442 catalyzed by the kinase GSK3β. Overexpression of FBXL19 abrogated the proapoptotic and inflammatory effects of IL-33 and lessened the severity of lung injury in mouse models of pneumonia. Our results suggest that modulation of the IL-33–ST2L axis by ubiquitin ligases might serve as a unique strategy for lessening pulmonary inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kurowska-Stolarska, M., Hueber, A., Stolarski, B. & McInnes, I.B. Interleukin-33: a novel mediator with a role in distinct disease pathologies. J. Intern. Med. 269, 29–35 (2011).
Smith, D.E. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin. Exp. Allergy 40, 200–208 (2011).
Küchler, A.M. et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am. J. Pathol. 173, 1229–1242 (2008).
Lüthi, A.U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
Talabot-Ayer, D., Lamacchia, C., Gabay, C. & Palmer, G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J. Biol. Chem. 284, 19420–19426 (2009).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Liu, X. et al. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 386, 181–185 (2009).
Ohno, T. et al. Paracrine IL-33 stimulation enhances lipopolysaccharide-mediated macrophage activation. PLoS ONE 6, e18404 (2011).
Espinassous, Q. et al. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J. Immunol. 183, 1446–1455 (2009).
Joshi, A.D. et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 11, 52 (2011).
Yin, H. et al. Adenovirus-mediated overexpression of soluble ST2 provides a protective effect on lipopolysaccharide-induced acute lung injury in mice. Clin. Exp. Immunol. 164, 248–255 (2011).
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. USA 107, 18581–18586 (2011).
Alves-Filho, J.C. et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 16, 708–712 (2011).
Li, H. et al. The cloning and nucleotide sequence of human ST2L cDNA. Genomics 67, 284–290 (2000).
Trajkovic, V., Sweet, M.J. & Xu, D. T1/ST2–an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 15, 87–95 (2004).
Sweet, M.J. et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J. Immunol. 166, 6633–6639 (2001).
Choi, Y.S. et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 114, 3117–3126 (2009).
Kurowska-Stolarska, M. et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 181, 4780–4790 (2008).
Kurowska-Stolarska, M. et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477 (2009).
Yagami, A. et al. IL-33 mediates inflammatory responses in human lung tissue cells. J. Immunol. 185, 5743–5750 (2011).
Rocca, A., Lamaze, C., Subtil, A. & Dautry-Varsat, A. Involvement of the ubiquitin/proteasome system in sorting of the interleukin 2 receptor beta chain to late endocytic compartments. Mol. Biol. Cell 12, 1293–1301 (2001).
Gesbert, F., Malarde, V. & Dautry-Varsat, A. Ubiquitination of the common cytokine receptor γc and regulation of expression by an ubiquitination/deubiquitination machinery. Biochem. Biophys. Res. Commun. 334, 474–480 (2005).
Martinez-Moczygemba, M., Huston, D.P. & Lei, J.T. JAK kinases control IL-5 receptor ubiquitination, degradation, and internalization. J. Leukoc. Biol. 81, 1137–1148 (2007).
Wauman, J., De Ceuninck, L., Vanderroost, N., Lievens, S. & Tavernier, J. RNF41 (Nrdp1) controls type 1 cytokine receptor degradation and ectodomain shedding. J. Cell Sci. 124, 921–932 (2011).
Jadhav, T. & Wooten, M.W. Defining an embedded code for protein ubiquitination. J. Proteomics Bioinform. 2, 316 (2009).
Nandi, D., Tahiliani, P., Kumar, A. & Chandu, D. The ubiquitin-proteasome system. J. Biosci. 31, 137–155 (2006).
Skowyra, D., Craig, K.L., Tyers, M., Elledge, S.J. & Harper, J.W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 (1997).
Katoh, M. & Katoh, M. Identification and characterization of FBXL19 gene in silico. Int. J. Mol. Med. 14, 1109–1114 (2004).
Carpenter, G. & Cohen, S. Epidermal growth factor. J. Biol. Chem. 265, 7709–7712 (1990).
Leng, S. et al. Glycogen synthase kinase 3 β mediates high glucose-induced ubiquitination and proteasome degradation of insulin receptor substrate 1. J. Endocrinol. 206, 171–181 (2010).
Zou, C. et al. LPS impairs phospholipid synthesis by triggering β-transducin repeat-containing protein (β-TrCP)-mediated polyubiquitination and degradation of the surfactant enzyme acyl-CoA:lysophosphatidylcholine acyltransferase I (LPCAT1). J. Biol. Chem. 286, 2719–2727 (2011).
Akcay, A. et al. IL-33 exacerbates acute kidney injury. J. Am. Soc. Nephrol. 22, 2057–2067 (2011).
Na, H.J., Hudson, S.A. & Bochner, B.S. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine 57, 169–174 (2012).
Chen, Y.R., Kori, R., John, B. & Tan, T.H. Caspase-mediated cleavage of actin-binding and SH3-domain-containing proteins cortactin, HS1, and HIP-55 during apoptosis. Biochem. Biophys. Res. Commun. 288, 981–989 (2001).
Timpson, P. et al. Aberrant expression of cortactin in head and neck squamous cell carcinoma cells is associated with enhanced cell proliferation and resistance to the epidermal growth factor receptor inhibitor gefitinib. Cancer Res. 67, 9304–9314 (2007).
Clark, E.S. et al. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene 28, 431–444 (2009).
Xu, D. et al. IL-33 exacerbates autoantibody-induced arthritis. J. Immunol. 184, 2620–2626 (2010).
Xu, D. et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. USA 105, 10913–10918 (2008).
Rong, Z. et al. Interleukin-17F signaling requires ubiquitination of interleukin-17 receptor via TRAF6. Cell. Signal. 19, 1514–1520 (2007).
Nguyen, C.Q., Yin, H., Lee, B.H., Chiorini, J.A. & Peck, A.B. IL17: potential therapeutic target in Sjogren's syndrome using adenovirus-mediated gene transfer. Lab. Invest. 91, 54–62 (2011).
Mueller, S.G., Schraw, W.P. & Richmond, A. Activation of protein kinase C enhances the phosphorylation of the type B interleukin-8 receptor and stimulates its degradation in non-hematopoietic cells. J. Biol. Chem. 270, 10439–10448 (1995).
Rottmann, S., Wang, Y., Nasoff, M., Deveraux, Q.L. & Quon, K.C.A. TRAIL receptor-dependent synthetic lethal relationship between MYC activation and GSK3beta/FBW7 loss of function. Proc. Natl. Acad. Sci. USA 102, 15195–15200 (2005).
Plotnikov, A. et al. Oncogene-mediated inhibition of glycogen synthase kinase 3β impairs degradation of prolactin receptor. Cancer Research. 68, 1354–1361 (2008).
Oboki, K., Nakae, S., Matsumoto, K. & Saito, H. IL-33 and airway inflammation. Allergy Asthma Immunol. Res. 3, 81–88 (2011).
Acknowledgements
We thank L. Wallace for technical assistance. This study is based on work supported in part by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. Supported by the US Department of Veterans Affairs (Merit Review Award), the US National Institutes of Health (R01 HL01916 to Y.Z. and R01 HL096376, R01 HL097376 and R01 HL098174 to R.K.M.) and the American Heart Association (12SDG9050005 to J.Z.). The contents presented do not represent the views of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
J.Z. and Y.Z. designed the study, did experiments, analyzed the data and wrote the manuscript; J.W., R.K.Mi. and D.F.M. did experiments; B.B.C. and T.C. cloned FBXL19; B.B.C. assisted with animal experiments; C.Z. provided reagents; and R.K.Ma. assisted Y.Z. with direction and study design and provided reagents and editorial assistance with this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 865 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Wei, J., Mialki, R. et al. F-box protein FBXL19–mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat Immunol 13, 651–658 (2012). https://doi.org/10.1038/ni.2341
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2341
This article is cited by
-
The deubiquitinase USP40 preserves endothelial integrity by targeting the heat shock protein HSP90β
Experimental & Molecular Medicine (2024)
-
Decreased ubiquitin modifying enzyme A20 associated with hyper-responsiveness to ovalbumin challenge following intrauterine growth restriction
Respiratory Research (2023)
-
Protective role of FBXL19 in Streptococcus pneumoniae-induced lung injury in pneumonia immature mice
Journal of Cardiothoracic Surgery (2023)
-
Pinpointing novel risk loci for Lewy body dementia and the shared genetic etiology with Alzheimer’s disease and Parkinson’s disease: a large-scale multi-trait association analysis
BMC Medicine (2022)
-
Osteocytes directly regulate osteolysis via MYD88 signaling in bacterial bone infection
Nature Communications (2022)