Abstract
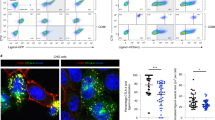
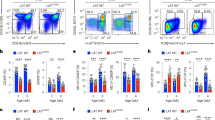
The aminopeptidase ERAAP is essential for trimming peptides presented by major histocompatibility complex (MHC) class I molecules. Inhibition of ERAAP by cytomegalovirus results in evasion of the immune response by this virus, and polymorphisms in ERAAP are associated with autoimmune disorders. How normal ERAAP function is monitored is unknown. We found that inhibition of ERAAP rapidly induced presentation of the peptide FYAEATPML (FL9) by the MHC class Ib molecule Qa-1b. Antigen-experienced T cells specific for the Qa-1b–FL9 complex were frequent in naive mice. Wild-type mice immunized with ERAAP-deficient cells mounted a potent CD8+ T cell response specific for Qa-1b–FL9. MHC class Ib–restricted cytolytic effector cells specifically eliminated ERAAP-deficient cells in vitro and in vivo. Thus, nonclassical Qa-1b–peptide complexes direct cytotoxic T cells to targets with defective antigen processing in the endoplasmic reticulum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shastri, N., Schwab, S. & Serwold, T. Producing nature's gene-chips: the generation of peptides for display by MHC class I molecules. Annu. Rev. Immunol. 20, 463–493 (2002).
Williams, M.A. & Bevan, M.J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
Peaper, D.R. & Cresswell, P. Regulation of MHC class I assembly and peptide binding. Annu. Rev. Cell Dev. Biol. 24, 343–368 (2008).
Serwold, T., Gonzalez, F., Kim, J., Jacob, R. & Shastri, N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419, 480–483 (2002).
Rodgers, J.R. & Cook, R.G. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 5, 459–471 (2005).
Strong, R.K. et al. HLA-E allelic variants. Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J. Biol. Chem. 278, 5082–5090 (2003).
Hansen, T.H. & Bouvier, M. MHC class I antigen presentation: learning from viral evasion strategies. Nat. Rev. Immunol. 9, 503–513 (2009).
Tortorella, D., Gewurz, B.E., Furman, M.H., Schust, D.J. & Ploegh, H.L. Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861–926 (2000).
Van Kaer, L., Ashton-Rickardt, P.G., Ploegh, H.L. & Tonegawa, S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+ T cells. Cell 71, 1205–1214 (1992).
Fruci, D. et al. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J. Cell Physiol. 216, 742–749 (2008).
Kim, S. et al. Human cytomegalovirus microRNA miR-US4–1 inhibits CD8+ T cell responses by targeting the aminopeptidase ERAP1. Nat. Immunol. 12, 984–991 (2011).
Evans, D.M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 (2011).
Strange, A. et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 42, 985–990 (2010).
Hammer, G.E., Gonzalez, F., Champsaur, M., Cado, D. & Shastri, N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat. Immunol. 7, 103–112 (2006).
Yan, J. et al. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 203, 647–659 (2006).
York, I.A., Brehm, M.A., Zendzian, S., Towne, C.F. & Rock, K.L. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc. Natl. Acad. Sci. USA 103, 9202–9207 (2006).
Hammer, G.E., Gonzalez, F., James, E., Nolla, H. & Shastri, N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat. Immunol. 8, 101–108 (2007).
Vugmeyster, Y. et al. Major histocompatibility complex (MHC) class I KbDb−/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc. Natl. Acad. Sci. USA 95, 12492–12497 (1998).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Zijlstra, M. et al. β2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature 344, 742–746 (1990).
Sanderson, S. & Shastri, N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 6, 369–376 (1994).
Hu, D. et al. Analysis of regulatory CD8+ T cells in Qa-1-deficient mice. Nat. Immunol. 5, 516–523 (2004).
Serwold, T., Gaw, S. & Shastri, N. ER aminopeptidases generate a unique pool of peptides for MHC class I molecules. Nat. Immunol. 2, 644–651 (2001).
Lu, L., Werneck, M.B. & Cantor, H. The immunoregulatory effects of Qa-1. Immunol. Rev. 212, 51–59 (2006).
Aldrich, C.J. et al. Identification of a TAP-dependent leader peptide recognized by alloreactive T cells specific for a class 1b antigen. Cell 79, 649–658 (1994).
Karttunen, J., Sanderson, S. & Shastri, N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. USA 89, 6020–6024 (1992).
Mach, N. et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60, 3239–3246 (2000).
Falk, K., Rötzschke, O. & Rammensee, H.G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature 348, 248–251 (1990).
Malarkannan, S., Goth, S., Buchholz, D.R. & Shastri, N. The role of MHC class I molecules in the generation of endogenous peptide/MHC complexes. J. Immunol. 154, 585–598 (1995).
Moon, J.J. et al. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 (2007).
Obar, J.J., Khanna, K.M. & Lefrancois, L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity 28, 859–869 (2008).
Moon, J.J. et al. Tracking epitope-specific T cells. Nat. Protoc. 4, 565–581 (2009).
Haluszczak, C. et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 206, 435–448 (2009).
Kerksiek, K.M., Busch, D.H., Pilip, I.M., Allen, S.E. & Pamer, E.G. H2–M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 190, 195–204 (1999).
Cho, H., Choi, H.J., Xu, H., Felio, K. & Wang, C.R. Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2–M3. J. Immunol. 186, 489–498 (2011).
Bouwer, H.G., Barry, R.A. & Hinrichs, D.J. Lack of expansion of major histocompatibility complex class Ib-restricted effector cells following recovery from secondary infection with the intracellular pathogen Listeria monocytogenes. Infect. Immun. 69, 2286–2292 (2001).
Cifaldi, L. et al. Natural killer cells efficiently reject lymphoma silenced for the endoplasmic reticulum aminopeptidase associated with antigen processing. Cancer Res. 71, 1597–1606 (2011).
Blanchard, N. et al. Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. J. Immunol. 184, 3033–3042 (2010).
Petroziello, J. et al. Suppression subtractive hybridization and expression profiling identifies a unique set of genes overexpressed in non-small-cell lung cancer. Oncogene 23, 7734–7745 (2004).
Gilli, F. et al. Learning from nature: pregnancy changes the expression of inflammation-related genes in patients with multiple sclerosis. PLoS ONE 5, e8962 (2010).
Yamagata, T., Benoist, C. & Mathis, D. A shared gene-expression signature in innate-like lymphocytes. Immunol. Rev. 210, 52–66 (2006).
Urdahl, K.B., Sun, J.C. & Bevan, M.J. Positive selection of MHC class Ib-restricted CD8+ T cells on hematopoietic cells. Nat. Immunol. 3, 772–779 (2002).
Cho, H., Bediako, Y., Xu, H., Choi, H.J. & Wang, C.R. Positive selecting cell type determines the phenotype of MHC class Ib-restricted CD8+ T cells. Proc. Natl. Acad. Sci. USA 108, 13241–13246 (2011).
Oliveira, C.C. et al. The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigen-processing defects. J. Exp. Med. 207, 207–221 (2010).
Acknowledgements
We thank the Tetramer Core Facility of the US National Institutes of Health for tetramer reagents; K. Söderstrom and E. Engleman (Stanford University) for Qa-1b-deficient mice generated by H. Cantor and colleagues (Harvard University); H. Cantor (Harvard University) for lentiviral vectors expressing Qa-1b; D. King for peptide synthesis; H. Nolla for assistance with cell sorting; K.C. Lind and A.H. Bakker for discussions and comments on the manuscript; and H. Dani for technical assistance. Supported by Irvington Institute Fellowship Program of the Cancer Research Institute (N.A.N.) and the US National Institutes of Health (N.S.).
Author information
Authors and Affiliations
Contributions
N.A.N. and N.S. designed the study and wrote the manuscript; and N.A.N. and F.G. did the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1458 kb)
Rights and permissions
About this article
Cite this article
Nagarajan, N., Gonzalez, F. & Shastri, N. Nonclassical MHC class Ib–restricted cytotoxic T cells monitor antigen processing in the endoplasmic reticulum. Nat Immunol 13, 579–586 (2012). https://doi.org/10.1038/ni.2282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2282
This article is cited by
-
Non-mutational neoantigens in disease
Nature Immunology (2024)
-
FAM49B promotes breast cancer proliferation, metastasis, and chemoresistance by stabilizing ELAVL1 protein and regulating downstream Rab10/TLR4 pathway
Cancer Cell International (2021)
-
Evidence of a tolerogenic vaccine against AIDS in the Chinese macaque prefigures a potential human vaccine
Archives of Virology (2021)
-
Editing the immunopeptidome of melanoma cells using a potent inhibitor of endoplasmic reticulum aminopeptidase 1 (ERAP1)
Cancer Immunology, Immunotherapy (2019)
-
ERAP1 promotes Hedgehog-dependent tumorigenesis by controlling USP47-mediated degradation of βTrCP
Nature Communications (2019)