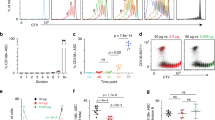
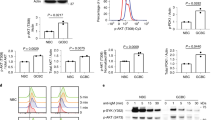
Abstract
Clonal deletion of autoreactive B cells is crucial for the prevention of autoimmunity, but the signaling mechanisms that regulate this checkpoint remain undefined. Here we characterize a previously unrecognized Ca2+-driven pathway for activation of the kinase Erk, which was proapoptotic and biochemically distinct from Erk activation induced by diacylglycerol (DAG). This pathway required protein kinase C-δ (PKC-δ) and the guanine nucleotide–exchange factor RasGRP and depended on the concentration of the Ca2+ sensor STIM1, which controls the magnitude of Ca2+ entry. Developmental regulation of these proteins was associated with selective activation of the pathway in B cells prone to negative selection. This checkpoint was impaired in PKC-δ-deficient mice, which developed B cell autoimmunity. Conversely, overexpression of STIM1 conferred a competitive disadvantage to developing B cells. Our findings establish Ca2+-dependent Erk signaling as a critical proapoptotic pathway that mediates the negative selection of B cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Gay, D., Saunders, T., Camper, S. & Weigert, M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177, 999–1008 (1993).
Goodnow, C.C. et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334, 676–682 (1988).
Nemazee, D.A. & Burki, K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337, 562–566 (1989).
Tiegs, S.L., Russell, D.M. & Nemazee, D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177, 1009–1020 (1993).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Hertz, M. & Nemazee, D. BCR ligation induces receptor editing in IgM+IgD− bone marrow B cells in vitro. Immunity 6, 429–436 (1997).
Melamed, D., Benschop, R.J., Cambier, J.C. & Nemazee, D. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell 92, 173–182 (1998).
Benschop, R.J., Brandl, E., Chan, A.C. & Cambier, J.C. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J. Immunol. 167, 4172–4179 (2001).
Gross, A.J., Lyandres, J.R., Panigrahi, A.K., Prak, E.T. & DeFranco, A.L. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J. Immunol. 182, 5382–5392 (2009).
Hoek, K.L. et al. Transitional B cell fate is associated with developmental stage-specific regulation of diacylglycerol and calcium signaling upon B cell receptor engagement. J. Immunol. 177, 5405–5413 (2006).
King, L.B., Norvell, A. & Monroe, J.G. Antigen receptor-induced signal transduction imbalances associated with the negative selection of immature B cells. J. Immunol. 162, 2655–2662 (1999).
Parekh, A.B. & Putney, J.W. Jr. Store-operated calcium channels. Physiol. Rev. 85, 757–810 (2005).
Liou, J. et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 (2005).
Roos, J. et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 (2005).
Zhang, S.L. et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 (2005).
Liou, J., Fivaz, M., Inoue, T. & Meyer, T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. USA 104, 9301–9306 (2007).
Luik, R.M., Wu, M.M., Buchanan, J. & Lewis, R.S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174, 815–825 (2006).
Park, C.Y. et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009).
Spassova, M.A. et al. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc. Natl. Acad. Sci. USA 103, 4040–4045 (2006).
Wu, M.M., Buchanan, J., Luik, R.M. & Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803–813 (2006).
Mecklenbrauker, I., Saijo, K., Zheng, N.Y., Leitges, M. & Tarakhovsky, A. Protein kinase Cδ controls self-antigen-induced B-cell tolerance. Nature 416, 860–865 (2002).
Miyamoto, A. et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature 416, 865–869 (2002).
Dower, N.A. et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1, 317–321 (2000).
Roose, J. & Weiss, A. T cells: getting a GRP on Ras. Nat. Immunol. 1, 275–276 (2000).
Roose, J.P., Mollenauer, M., Gupta, V.A., Stone, J. & Weiss, A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 25, 4426–4441 (2005).
Teixeira, C., Stang, S.L., Zheng, Y., Beswick, N.S. & Stone, J.C. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102, 1414–1420 (2003).
Downward, J., Graves, J.D., Warne, P.H., Rayter, S. & Cantrell, D.A. Stimulation of p21ras upon T-cell activation. Nature 346, 719–723 (1990).
Zhong, X.P. et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 4, 882–890 (2003).
Das, J. et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell 136, 337–351 (2009).
Ebinu, J.O. et al. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082–1086 (1998).
Ebinu, J.O. et al. RasGRP links T-cell receptor signaling to Ras. Blood 95, 3199–3203 (2000).
Oh-hora, M., Johmura, S., Hashimoto, A., Hikida, M. & Kurosaki, T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp. Med. 198, 1841–1851 (2003).
Roose, J.P., Mollenauer, M., Ho, M., Kurosaki, T. & Weiss, A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol. Cell. Biol. 27, 2732–2745 (2007).
Guilbault, B. & Kay, R.J. RasGRP1 sensitizes an immature B cell line to antigen receptor-induced apoptosis. J. Biol. Chem. 279, 19523–19530 (2004).
Stang, S.L. et al. A proapoptotic signaling pathway involving RasGRP, Erk, and Bim in B cells. Exp. Hematol. 37, 122–134 (2009).
Aiba, Y. et al. Activation of RasGRP3 by phosphorylation of Thr-133 is required for B cell receptor-mediated Ras activation. Proc. Natl. Acad. Sci. USA 101, 16612–16617 (2004).
Zheng, Y. et al. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood 105, 3648–3654 (2005).
Martiny-Baron, G. et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268, 9194–9197 (1993).
Blom, N., Gammeltoft, S. & Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362 (1999).
Eswar, N. et al. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 31, 3375–3380 (2003).
Freedman, T.S. et al. A Ras-induced conformational switch in the Ras activator Son of sevenless. Proc. Natl. Acad. Sci. USA 103, 16692–16697 (2006).
Mecklenbrauker, I., Kalled, S.L., Leitges, M., Mackay, F. & Tarakhovsky, A. Regulation of B-cell survival by BAFF-dependent PKCδ-mediated nuclear signalling. Nature 431, 456–461 (2004).
Steinberg, S.F. Distinctive activation mechanisms and functions for protein kinase Cδ. Biochem. J. 384, 449–459 (2004).
Yoshida, K. PKCδ signaling: mechanisms of DNA damage response and apoptosis. Cell. Signal. 19, 892–901 (2007).
Hartley, S.B. et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell 72, 325–335 (1993).
Hartley, S.B. et al. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353, 765–769 (1991).
Freedman, T.S. et al. Differences in flexibility underlie functional differences in the Ras activators son of sevenless and Ras guanine nucleotide releasing factor 1. Structure 17, 41–53 (2009).
Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6, 827–837 (2005).
Murphy, L.O. & Blenis, J. MAPK signal specificity: the right place at the right time. Trends Biochem. Sci. 31, 268–275 (2006).
Philips, M.R. Compartmentalized signalling of Ras. Biochem. Soc. Trans. 33, 657–661 (2005).
Daniels, M.A. et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 (2006).
Acknowledgements
We thank the Sandler-Moore Mass Spectrometry Core Facility at the University of California at San Francisco (funded by the Sandler Family Foundation, the Gordon and Betty Moore Foundation and the National Cancer Institute of the US National Institutes of Health (P30 CA82103)) for assistance in protein identification; the Flow Cytometry Core Facility at the Department of Pathology and Diabetes Center of the University of California at San Francisco for assistance; G. Koretzky (University of Pennsylvania) for the pEF-Flag-hDGK-ζ plasmid; A. Roque for assistance with animal husbandry; H. Phee and M. Hermiston for critical reading of the manuscript and suggestions; B. Au-Yeung and H. Wang for help with tail-vein injections; and members of the Weiss laboratory for discussions. Supported by the Howard Hughes Medical Institute and the Sidney Kimmel Foundation (J.P.R.).
Author information
Authors and Affiliations
Contributions
A.L. designed and did experiments, analyzed the data and wrote the manuscript; P.D. collaborated with A.L. in determining expression of various proteins on sorted bone marrow B cell populations and in collecting reconstituted mice; T.S.F. did homology modeling of RasGRP1; J.L. and T.K. provided reagents; M.L. generated the Prkcd−/− mice; J.P.R. designed experiments and provided reagents; and A.W. designed experiments, supervised the research, revised the manuscript and provided support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 706 kb)
Rights and permissions
About this article
Cite this article
Limnander, A., Depeille, P., Freedman, T. et al. STIM1, PKC-δ and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat Immunol 12, 425–433 (2011). https://doi.org/10.1038/ni.2016
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2016
This article is cited by
-
RasGRP1 promotes the acute inflammatory response and restricts inflammation-associated cancer cell growth
Nature Communications (2022)
-
CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes
Cellular & Molecular Immunology (2018)
-
Autoimmunity checkpoints as therapeutic targets in B cell malignancies
Nature Reviews Cancer (2018)
-
RAS at the Golgi antagonizes malignant transformation through PTPRκ-mediated inhibition of ERK activation
Nature Communications (2018)
-
Differential effects of FTY720 on the B cell compartment in a mouse model of multiple sclerosis
Journal of Neuroinflammation (2017)