Abstract
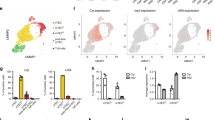
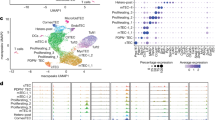
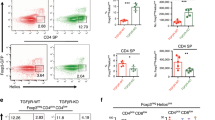
Medullary thymic epithelial cells (mTECs) serve an essential function in central tolerance by expressing peripheral-tissue antigens. These antigens may be transferred to and presented by dendritic cells (DCs). Therefore, it is unclear whether mTECs, in addition to being an antigen reservoir, also serve a mandatory function as antigen-presenting cells. Here we diminished major histocompatibility complex (MHC) class II on mTECs through transgenic expression of a 'designer' microRNA specific for the MHC class II transactivator CIITA (called 'C2TA' here). This resulted in an enlarged polyclonal CD4+ single-positive compartment and, among thymocytes specific for model antigens expressed in mTECs, enhanced selection of regulatory T cells (Treg cells) at the expense of deletion. Our data document an autonomous contribution of mTECs to both dominant and recessive mechanisms of CD4+ T cell tolerance and support an avidity model of Treg cell development versus deletion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Hogquist, K.A., Baldwin, T.A. & Jameson, S.C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782 (2005).
Klein, L., Hinterberger, M., Wirnsberger, G. & Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 9, 833–844 (2009).
Ohnmacht, C. et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 206, 549–559 (2009).
van Meerwijk, J.P. et al. Quantitative impact of thymic clonal deletion on the T cell repertoire. J. Exp. Med. 185, 377–383 (1997).
Akiyama, T. et al. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science 308, 248–251 (2005).
Akiyama, T. et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29, 423–437 (2008).
Hikosaka, Y. et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29, 438–450 (2008).
Kajiura, F. et al. NF-κB-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J. Immunol. 172, 2067–2075 (2004).
Kinoshita, D. et al. Essential role of IκB kinase α in thymic organogenesis required for the establishment of self-tolerance. J. Immunol. 176, 3995–4002 (2006).
Rossi, S.W. et al. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204, 1267–1272 (2007).
Mathis, D. & Benoist, C. Aire. Annu. Rev. Immunol. 27, 287–312 (2009).
Peterson, P., Org, T. & Rebane, A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat. Rev. Immunol. 8, 948–957 (2008).
Derbinski, J. et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202, 33–45 (2005).
Anderson, M.S. et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002).
Gallegos, A.M. & Bevan, M.J. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 200, 1039–1049 (2004).
Millet, V., Naquet, P. & Guinamard, R.R. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur. J. Immunol. 38, 1257–1263 (2008).
Koble, C. & Kyewski, B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J. Exp. Med. 206, 1505–1513 (2009).
Gray, D., Abramson, J., Benoist, C. & Mathis, D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 204, 2521–2528 (2007).
Aschenbrenner, K. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 (2007).
Jordan, M.S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001).
Liston, A. & Rudensky, A.Y. Thymic development and peripheral homeostasis of regulatory T cells. Curr. Opin. Immunol. 19, 176–185 (2007).
Irla, M. et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29, 451–463 (2008).
Reith, W., LeibundGut-Landmann, S. & Waldburger, J.M. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 5, 793–806 (2005).
Zeng, Y., Wagner, E.J. & Cullen, B.R. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 9, 1327–1333 (2002).
Stegmeier, F., Hu, G., Rickles, R.J., Hannon, G.J. & Elledge, S.J. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 102, 13212–13217 (2005).
Dickins, R.A. et al. Tissue-specific and reversible RNA interference in transgenic mice. Nat. Genet. 39, 914–921 (2007).
Dickins, R.A. et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37, 1289–1295 (2005).
Paddison, P.J. et al. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat. Methods 1, 163–167 (2004).
Gardner, J.M. et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321, 843–847 (2008).
Otten, L.A. et al. Revisiting the specificity of the MHC class II transactivator CIITA in vivo. Eur. J. Immunol. 36, 1548–1558 (2006).
LeibundGut-Landmann, S. et al. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 34, 1513–1525 (2004).
Wu, L. & Shortman, K. Heterogeneity of thymic dendritic cells. Semin. Immunol. 17, 304–312 (2005).
Klein, L., Khazaie, K. & von Boehmer, H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA 100, 8886–8891 (2003).
Klein, L., Roettinger, B. & Kyewski, B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur. J. Immunol. 31, 2476–2486 (2001).
Nedjic, J., Aichinger, M., Emmerich, J., Mizushima, N. & Klein, L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455, 396–400 (2008).
Oukka, M. et al. CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium. Immunity 4, 545–553 (1996).
Li, J., Park, J., Foss, D. & Goldschneider, I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J. Exp. Med. 206, 607–622 (2009).
Proietto, A.I. et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA 105, 19869–19874 (2008).
Bensinger, S.J., Bandeira, A., Jordan, M.S., Caton, A.J. & Laufer, T.M. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 194, 427–438 (2001).
Liston, A. et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc. Natl. Acad. Sci. USA 105, 11903–11908 (2008).
Spence, P.J. & Green, E.A. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc. Natl. Acad. Sci. USA 105, 973–978 (2008).
Wirnsberger, G., Mair, F. & Klein, L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc. Natl. Acad. Sci. USA 106, 10278–10283 (2009).
Lio, C.W. & Hsieh, C.S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Bautista, J.L. et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10, 610–617 (2009).
Leung, M.W., Shen, S. & Lafaille, J.J. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J. Exp. Med. 206, 2121–2130 (2009).
Derbinski, J., Pinto, S., Rosch, S., Hexel, K. & Kyewski, B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc. Natl. Acad. Sci. USA 105, 657–662 (2008).
Venanzi, E.S., Melamed, R., Mathis, D. & Benoist, C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc. Natl. Acad. Sci. USA 105, 15860–15865 (2008).
Grusby, M.J., Johnson, R.S., Papaioannou, V.E. & Glimcher, L.H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253, 1417–1420 (1991).
Chang, C.H., Guerder, S., Hong, S.C., van Ewijk, W. & Flavell, R.A. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity 4, 167–178 (1996).
Kirberg, J. et al. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 180, 25–34 (1994).
Murphy, K.M., Heimberger, A.B. & Loh, D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250, 1720–1723 (1990).
Bot, A., Bot, S., Antohi, S., Karjalainen, K. & Bona, C. Kinetics of generation and persistence on membrane class II molecules of a viral peptide expressed on foreign and self proteins. J. Immunol. 157, 3436–3442 (1996).
Gentleman, R.C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Smyth, G.K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3 Article 3 (2004).
Acknowledgements
We thank B. Kyewski for critical reading of the manuscript; C. Federle and S. Hoeflinger for technical assistance; C. Theussl for BAC injections; M.S. Anderson (University of California, San Francisco) for Adig mice; K. Karjalainen (Nanyang Technological University) for A5 hybridoma cells; and H.S. Scott (University of Adelaide) for monoclonal antibody to Aire. Supported by the Deutsche Forschungsgemeinschaft (KL 1228/3-1 to L.K. and M.H.; SFB 455 to L.K. and M.A.; and VO 944/2-2 to D.V.) and the Austrian National Science Fund (SFB23).
Author information
Authors and Affiliations
Contributions
M.H. generated and analyzed the C2TAkd model as well as the compound transgenic mice and bone marrow chimeras and was also essentially involved in all other experiments; M.A. did expression analyses by quantitative PCR; O.P.d.C. and R.H. did microarray experiments; D.V. provided ΔDC mice; and L.K. and M.H. designed experimental strategies and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1321 kb)
Rights and permissions
About this article
Cite this article
Hinterberger, M., Aichinger, M., Prazeres da Costa, O. et al. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat Immunol 11, 512–519 (2010). https://doi.org/10.1038/ni.1874
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1874