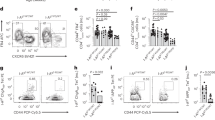
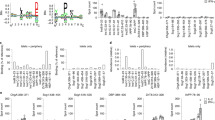
Abstract
In addition to the genetic framework, there are two other critical requirements for the development of tissue-specific autoimmune disease. First, autoreactive T cells need to escape thymic negative selection. Second, they need to find suitable conditions for autoantigen presentation and activation in the target tissue. We show here that these two conditions are fulfilled in diabetic mice of the nonobese diabetic (NOD) strain. A set of autoreactive CD4+ T cells specific for an insulin peptide, with the noteworthy feature of not recognizing the insulin protein when processed by antigen-presenting cells (APCs), escaped thymic control, participated in diabetes and caused disease. Moreover, APCs in close contact with beta cells in the islets of Langerhans bore vesicles with the antigenic insulin peptides and activated peptide-specific T cells. Our findings may be relevant for other cases of endocrine autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
05 March 2010
In the version of this article initially published online, some hybridomas in Figure 3 were misidentified. The correct text for Figure 3c is “type A (4F7) and type B (2D10 and 1.7) hybridomas” and the correct text for Figure 3e,f is “type B (2D10; e) and type A (4F7; f) hybridomas.” The error has been corrected for the print, PDF and HTML versions of this article.
References
Anderson, M.S. & Bluestone, J.A. The NOD mouse: a model of immune disregulation. Annu. Rev. Immunol. 23, 447–485 (2005).
Zhang, L., Nakayama, M. & Eisenbarth, G.S. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 20, 111–118 (2008).
Wegmann, D.R., Norbury-Glaser, M. & Daniel, D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur. J. Immunol. 24, 1853–1857 (1994).
Wegmann, D.R., Gill, R.G., Norbury-Glaser, M., Schloot, N. & Daniel, D. Analysis of the spontaneous T cell response to insulin in NOD mice. J. Autoimmun. 7, 833–843 (1994).
Daniel, D., Gill, R.G., Schloot, N. & Wegmann, D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 25, 1056–1062 (1995).
Moriyama, H. et al. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc. Natl. Acad. Sci. USA 100, 10376–10381 (2003).
Nakayama, M. et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005).
Jasinski, J.M. et al. Transgenic insulin (B:9–23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes 55, 1978–1984 (2006).
Chentoufi, A.A. & Polychronakos, C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes 51, 1383–1390 (2002).
Garcia, C.A. et al. Dendritic cells in human thymus and periphery display a proinsulin epitope in a transcription-dependent, capture-independent fashion. J. Immunol. 175, 2111–2122 (2005).
Pugliese, A. et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15, 293–297 (1997).
Pugliese, A. et al. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J. Clin. Invest. 107, 555–564 (2001).
French, M.B. et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes 46, 34–39 (1997).
Jaeckel, E., Lipes, M.A. & von Boehmer, H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat. Immunol. 5, 1028–1035 (2004).
Abiru, N. et al. Dual overlapping peptides recognized by insulin peptide B:9–23 T cell receptor AV13S3 T cell clones of the NOD mouse. J. Autoimmun. 14, 231–237 (2000).
Abiru, N. et al. Peptide and major histocompatibility complex-specific breaking of humoral tolerance to native insulin with the B9–23 peptide in diabetes-prone and normal mice. Diabetes 50, 1274–1281 (2001).
Nakayama, M. et al. Priming and effector dependence on insulin B:9–23 peptide in NOD islet autoimmunity. J. Clin. Invest. 117, 1835–1843 (2007).
Levisetti, M.G., Suri, A., Petzold, S.J. & Unanue, E.R. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J. Immunol. 178, 6051–6057 (2007).
Hausmann, D.H., Yu, B., Hausmann, S. & Wucherpfennig, K.W. pH-dependent peptide binding properties of the type I diabetes-associated I-Ag7 molecule: rapid release of CLIP at an endosomal pH. J. Exp. Med. 189, 1723–1734 (1999).
Levisetti, M.G., Lewis, D.M., Suri, A. & Unanue, E.R. Weak proinsulin peptide-MHC complexes are targeted in autoimmune diabetes in mice. Diabetes 57, 1852–1860 (2008).
Liu, G.Y. et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3, 407–415 (1995).
Fairchild, P.J., Wildgoose, R., Atherton, E., Webb, S. & Wraith, D.C. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int. Immunol. 5, 1151–1158 (1993).
Pu, Z., Lovitch, S.B., Bikoff, E.K. & Unanue, E.R. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity 20, 467–476 (2004).
Pu, Z., Carrero, J.A. & Unanue, E.R. Distinct recognition by two subsets of T cells of an MHC class II-peptide complex. Proc. Natl. Acad. Sci. USA 99, 8844–8849 (2002).
Peterson, D.A., DiPaolo, R.J., Kanagawa, O. & Unanue, E.R. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity 11, 453–462 (1999).
Lovitch, S.B., Walters, J.J., Gross, M.L. & Unanue, E.R. APCs present A beta(k)-derived peptides that are autoantigenic to type B T cells. J. Immunol. 170, 4155–4160 (2003).
Kawamura, K., McLaughlin, K.A., Weissert, R. & Forsthuber, T.G. Myelin-reactive type B T cells and T cells specific for low-affinity MHC-binding myelin peptides escape tolerance in HLA-DR transgenic mice. J. Immunol. 181, 3202–3211 (2008).
Calderon, B., Suri, A., Miller, M.J. & Unanue, E.R. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc. Natl. Acad. Sci. USA 105, 6121–6126 (2008).
Hutton, J.C. The insulin secretory granule. Diabetologia 32, 271–281 (1989).
Haskins, K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv. Immunol. 87, 123–162 (2005).
Dadaglio, G. et al. Characterization and quantification of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity 6, 727–738 (1997).
Hoglund, P. et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 189, 331–339 (1999).
Catalfamo, M. et al. HLA-DM and invariant chain are expressed by thyroid follicular cells, enabling the expression of compact DR molecules. Int. Immunol. 11, 269–277 (1999).
Croizet, K. et al. Culture of dendritic cells from a nonlymphoid organ, the thyroid gland: evidence for TNFα dependent phenotypic changes of thyroid-derived dendritic cells. Lab. Invest. 80, 1215–1225 (2000).
Gray, D. et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J. Immunol. Methods 329, 56–66 (2008).
Brunner, Y. et al. Proteomics analysis of insulin secretory granules. Mol. Cell. Proteomics 6, 1007–1017 (2007).
Acknowledgements
We thank P. Bittner and K. Frederick for technical assistance; R. Belizaire and M. Colonna for comments on the manuscript; M. Gross and H. Rohrs for mass spectrometry; K. Green for electron microscopy; G.S. Eisenbarth and N. Abiru (University of Colorado) for the M12.C3.G7β9-23 cell line; and E. Leiter (Jackson Laboratory) for NIT-1 cells. Supported by National Institutes of Health (AI024742, DK058177 and P60DK20579), the Juvenile Diabetes Research Foundation (JDRF 1-2007-731) and the Kilo Diabetes and Vascular Research Foundation.
Author information
Authors and Affiliations
Contributions
J.F.M., M.G.L. and E.R.U. designed and evaluated the experimental work; J.F.M., M.G.L. and J.W.H. did most of the cellular experiments; B.C. did immunofluorescence and confocal microscopy; S.J.P. isolated insulin granules for mass spectrometry and quantified insulin content in granules; and J.F.M. and E.R.U. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 398 kb)
Rights and permissions
About this article
Cite this article
Mohan, J., Levisetti, M., Calderon, B. et al. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol 11, 350–354 (2010). https://doi.org/10.1038/ni.1850
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1850
This article is cited by
-
The MHC-II peptidome of pancreatic islets identifies key features of autoimmune peptides
Nature Immunology (2020)
-
LAG-3 inhibits the activation of CD4+ T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII
Nature Immunology (2018)
-
Pancreatic islets communicate with lymphoid tissues via exocytosis of insulin peptides
Nature (2018)
-
The resident macrophages in murine pancreatic islets are constantly probing their local environment, capturing beta cell granules and blood particles
Diabetologia (2018)
-
Evolving Insights for MHC Class II Antigen Processing and Presentation in Health and Disease
Current Pharmacology Reports (2017)