Abstract
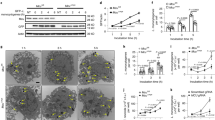
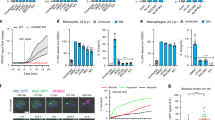
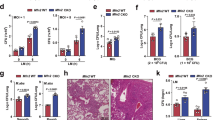
In response to invading microorganisms, macrophages engage in phagocytosis and rapidly release reactive oxygen species (ROS), which serve an important microbicidal function. However, how phagocytosis induces ROS production remains largely unknown. CARD9, a caspase-recruitment domain (CARD)-containing protein, is important for resistance to fungal and bacterial infection. The mechanism of CARD9-mediated bacterial clearance is still mostly unknown. Here we show that CARD9 is required for killing intracellular bacteria in macrophages. CARD9 associated with the GDP-dissociation inhibitor LyGDI in phagosomes after bacterial and fungal infection and binding of CARD9 suppressed LyGDI-mediated inhibition of the GTPase Rac1, thereby leading to ROS production and bacterial killing in macrophages. Thus, our studies identify a key pathway that leads to microbe-elicited ROS production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mosser, D.M. & Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Scott, C.C., Botelho, R.J. & Grinstein, S. Phagosome maturation: a few bugs in the system. J. Membr. Biol. 193, 137–152 (2003).
Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 (2004).
Sumimoto, H., Miyano, K. & Takeya, R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 338, 677–686 (2005).
Groemping, Y. & Rittinger, K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 386, 401–416 (2005).
Bokoch, G.M. & Diebold, B.A. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100, 2692–2696 (2002).
Heasman, S.J. & Ridley, A.J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 (2008).
Rossman, K.L., Der, C.J. & Sondek, J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 (2005).
Dovas, A. & Couchman, J.R. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 390, 1–9 (2005).
DerMardirossian, C. & Bokoch, G.M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
Bretscher, A., Edwards, K. & Fehon, R.G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 (2002).
Allenspach, E.J. et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15, 739–750 (2001).
Takahashi, K. et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem. 272, 23371–23375 (1997).
Janmey, P.A. & Lindberg, U. Cytoskeletal regulation: rich in lipids. Nat. Rev. Mol. Cell Biol. 5, 658–666 (2004).
Hsu, Y.M. et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat. Immunol. 8, 198–205 (2007).
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006).
Hara, H. et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat. Immunol. 8, 619–629 (2007).
Schnupf, P. & Portnoy, D.A. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9, 1176–1187 (2007).
Hamon, M., Bierne, H. & Cossart, P. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4, 423–434 (2006).
Myers, J.T., Tsang, A.W. & Swanson, J.A. Localized reactive oxygen and nitrogen intermediates inhibit escape of Listeria monocytogenes from vacuoles in activated macrophages. J. Immunol. 171, 5447–5453 (2003).
Ding, Z. et al. A retrovirus-based protein complementation assay screen reveals functional AKT1-binding partners. Proc. Natl. Acad. Sci. USA 103, 15014–15019 (2006).
Zal, T. Visualization of protein interactions in living cells. Adv. Exp. Med. Biol. 640, 183–197 (2008).
Kerppola, T.K. Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu Rev Biophys 37, 465–487 (2008).
Ciruela, F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr. Opin. Biotechnol. 19, 338–343 (2008).
Liu, D., Yang, X., Yang, D. & Songyang, Z. Genetic screens in mammalian cells by enhanced retroviral mutagens. Oncogene 19, 5964–5972 (2000).
Scherle, P., Behrens, T. & Staudt, L.M. Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc. Natl. Acad. Sci. USA 90, 7568–7572 (1993).
Lelias, J.M. et al. cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP-binding proteins. Proc. Natl. Acad. Sci. USA 90, 1479–1483 (1993).
Goodridge, H.S. et al. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154 (2009).
Pick, E., Gorzalczany, Y. & Engel, S. Role of the rac1 p21-GDP-dissociation inhibitor for rho heterodimer in the activation of the superoxide-forming NADPH oxidase of macrophages. Eur. J. Biochem. 217, 441–455 (1993).
Abo, A. et al. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353, 668–670 (1991).
Benard, V., Bohl, B.P. & Bokoch, G.M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198–13204 (1999).
Bokoch, G.M. Regulation of the phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 5, 109–113 (1995).
Shaughnessy, L.M. & Swanson, J.A. The role of the activated macrophage in clearing Listeria monocytogenes infection. Front. Biosci. 12, 2683–2692 (2007).
Blander, J.M. & Medzhitov, R. On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 7, 1029–1035 (2006).
Shiloh, M.U. et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10, 29–38 (1999).
Wells, C.A. et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 180, 7404–7413 (2008).
Yamasaki, S. et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9, 1179–1188 (2008).
Sato, K. et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J. Biol. Chem. 281, 38854–38866 (2006).
Himes, S.R., Cronau, S., Mulford, C. & Hume, D.A. The Runx 1 transcription factor controls CSF-1-dependent and -independent growth and survival of macrophages. Oncogene 24, 5278–5286 (2005).
Daniel, D.S. et al. The reduced bactericidal function of complement C5-deficient murine macrophages is associated with defects in the synthesis and delivery of reactive oxygen radicals to mycobacterial phagosomes. J. Immunol. 177, 4688–4698 (2006).
Acknowledgements
We thank W. Li and F. Southwick (University of Florida) for GFP–L. monocytogenes, and X. Qin (University of Texas MD Anderson Cancer Center) for the retroviral packaging vector pCGP and pVSV-G. Supported by the National Institutes of Health (RO1AI050848 and R01GM065899 to X.L. and R01GM069572 to Z.S.), the Welch Foundation (Z.S.) and the Leukemia and Lymphoma Society (X.L. and Z.S.).
Author information
Authors and Affiliations
Contributions
W.W., Y.-M.S.H. and L.B. did the experiments; W.W. and X.L. designed the experiments, analyzed the results and wrote the manuscript; and Z.S. provided the reagents and technical advice.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 626 kb)
Rights and permissions
About this article
Cite this article
Wu, W., Hsu, YM., Bi, L. et al. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat Immunol 10, 1208–1214 (2009). https://doi.org/10.1038/ni.1788
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1788
This article is cited by
-
Role of Rho GTPases in inflammatory bowel disease
Cell Death Discovery (2023)
-
Inflammation and Neutrophil Oxidative Burst in a Family with NFKB1 p.R157X LOF and Sterile Necrotizing Fasciitis
Journal of Clinical Immunology (2023)
-
DOCK2 regulates antifungal immunity by regulating RAC GTPase activity
Cellular & Molecular Immunology (2022)
-
Card9 protects sepsis by regulating Ripk2-mediated activation of NLRP3 inflammasome in macrophages
Cell Death & Disease (2022)
-
Caspase recruitment domain (CARD) family (CARD9, CARD10, CARD11, CARD14 and CARD15) are increased during active inflammation in patients with inflammatory bowel disease
Journal of Inflammation (2018)