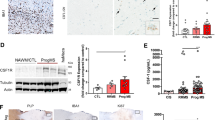
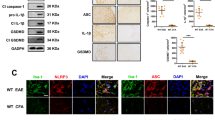
Abstract
Multiple sclerosis is an inflammatory disease of the central nervous system that begins as a relapsing-remitting disease (RRMS) and is followed by a progressive phase (SPMS). The progressive phase causes the greatest disability and has no effective therapy, but the processes that drive SPMS are mostly unknown. Here we found higher serum concentrations of 15α-hydroxicholestene (15-HC) in patients with SPMS and in mice with secondary progressive experimental autoimmune encephalomyelitis (EAE) but not in patients with RRMS. In mice, 15-HC activated microglia, macrophages and astrocytes through a pathway involving Toll-like receptor 2 (TLR2) and poly(ADP-ribose) polymerase 1 (PARP-1). PARP-1 activity was higher in monocytes of patients with SPMS, and PARP-1 inhibition suppressed the progression of EAE. Thus, the TLR2–PARP-1 pathway is a potential new therapeutic target in SPMS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
18 September 2009
NOTE: In the version of this article initially published, two citations were not included. These citations, together with text describing their content, have been added to page 958, column 2, as follows: “As neuronal loss is thought to contribute to the pathogenesis of progressive multiple sclerosis5, and as PARP-1 inhibitors suppress the incidence and severity of experimental autoimmune encephalomyelitis (EAE)36,37, we investigated the function of 15-oxysterols in multiple sclerosis and EAE.” The added references are as follows: 36. Scott, G.S. et al. Role of poly(ADP-ribose) synthetase activation in the development of experimental allergic encephalomyelitis. J. Neuroimmunol. 117, 78–86 (2001). 37. Scott, G.S. et al. The therapeutic effects of PJ34 [N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide.HCl], a selective inhibitor of poly(ADP-ribose) polymerase, in experimental allergic encephalomyelitis are associated with immunomodulation. J. Pharmacol. Exp. Ther. 310, 1053–1061 (2004). The error has been corrected in the HTML and PDF versions of the article.
References
McFarland, H.F. & Martin, R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 8, 913–919 (2007).
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Rovaris, M. et al. Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol. 5, 343–354 (2006).
Lopez-Diego, R.S. & Weiner, H.L. Novel therapeutic strategies for multiple sclerosis—a multifaceted adversary. Nat. Rev. Drug Discov. 7, 909–925 (2008).
Trapp, B.D. & Nave, K.A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 (2008).
Trapp, B.D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Balashov, K.E., Comabella, M., Ohashi, T., Khoury, S.J. & Weiner, H.L. Defective regulation of IFNγ and IL-12 by endogenous IL-10 in progressive MS. Neurology 55, 192–198 (2000).
Balashov, K.E., Smith, D.R., Khoury, S.J., Hafler, D.A. & Weiner, H.L. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc. Natl. Acad. Sci. USA 94, 599–603 (1997).
Comabella, M. et al. Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J. Clin. Invest. 102, 671–678 (1998).
Karni, A., Koldzic, D.N., Bharanidharan, P., Khoury, S.J. & Weiner, H.L. IL-18 is linked to raised IFN-γ in multiple sclerosis and is induced by activated CD4+ T cells via CD40–CD40 ligand interactions. J. Neuroimmunol. 125, 134–140 (2002).
Karni, A. et al. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 177, 4196–4202 (2006).
Weiner, H.L. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 255, 3–11 (2008).
Quintana, F.J. et al. Functional immunomics: microarray analysis of IgG autoantibody repertoires predicts the future response of mice to induced diabetes. Proc. Natl. Acad. Sci. USA 101, 14615–14621 (2004).
Robinson, W.H. et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 (2003).
Quintana, F.J. et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 105, 18889–18894 (2008).
Diestel, A. et al. Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: a link between demyelination and neuronal damage. J. Exp. Med. 198, 1729–1740 (2003).
Basso, A.S. et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 118, 1532–1543 (2008).
Suto, M.J., Turner, W.R., Arundel-Suto, C.M., Werbel, L.M. & Sebolt-Leopold, J.S. Dihydroisoquinolinones: the design and synthesis of a new series of potent inhibitors of poly(ADP-ribose) polymerase. Anticancer Drug Des. 6, 107–117 (1991).
Jack, C., Ruffini, F., Bar-Or, A. & Antel, J.P. Microglia and multiple sclerosis. J. Neurosci. Res. 81, 363–373 (2005).
Nair, A., Frederick, T.J. & Miller, S.D. Astrocytes in multiple sclerosis: a product of their environment. Cell. Mol. Life Sci. 65, 2702–2720 (2008).
Smith, K.J. & Lassmann, H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 1, 232–241 (2002).
Charo, I.F. & Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Kanter, J.L. et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 12, 138–143 (2006).
Chan, S.M., Ermann, J., Su, L., Fathman, C.G. & Utz, P.J. Protein microarrays for multiplex analysis of signal transduction pathways. Nat. Med. 10, 1390–1396 (2004).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Cohen-Armon, M. et al. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol. Cell 25, 297–308 (2007).
Leoni, V., Lutjohann, D. & Masterman, T. Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J. Lipid Res. 46, 191–195 (2005).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
Bongarzone, E.R., Pasquini, J.M. & Soto, E.F. Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. J. Neurosci. Res. 41, 213–221 (1995).
Luo, J. et al. Glia-dependent TGF-β signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J. Clin. Invest. 117, 3306–3315 (2007).
Pitt, D., Werner, P. & Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6, 67–70 (2000).
Smith, T., Groom, A., Zhu, B. & Turski, L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 6, 62–66 (2000).
Quintana, F.J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Meng, G. et al. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Invest. 113, 1473–1481 (2004).
Qi, H.Y. & Shelhamer, J.H. Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 280, 38969–38975 (2005).
Scott, G.S. et al. Role of poly(ADP-ribose) synthetase activation in the development of experimental allergic encephalomyelitis. J. Neuroimmunol. 117, 78–86 (2001).
Scott, G.S et al. The therapeutic effects of PJ34 [N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide.HCl], a selective inhibitor of poly(ADP-ribose) polymerase, in experimental allergic encephalomyelitis are associated with immunomodulation. J. Pharmacol. Exp. Ther. 310, 1053–1061 (2004).
Acknowledgements
We thank A.S. Basso (Universidade Federal de São Paulo) for discussions, and M. Oukka (Harvard Medical School) for TLR2-deficient mice. Supported by the US National Institutes of Health (AI435801 and NS38037), the US National Multiple Sclerosis Society (PP1289 to H.L.W.) and the Human Frontiers of Science Program Organization (F.J.Q.).
Author information
Authors and Affiliations
Contributions
M.F.F and F.J.Q. did experiments and analyzed data; R.G. provided purified human monocytes; G.I and M.L. contributed samples; and M.F.F., F.J.Q. and H.L.W. wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–6 (PDF 955 kb)
Rights and permissions
About this article
Cite this article
Farez, M., Quintana, F., Gandhi, R. et al. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol 10, 958–964 (2009). https://doi.org/10.1038/ni.1775
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1775
This article is cited by
-
Chi3l3 induces oligodendrogenesis in an experimental model of autoimmune neuroinflammation
Nature Communications (2019)
-
Microglia Receptors in Animal Models of Traumatic Brain Injury
Molecular Neurobiology (2019)
-
PARP inhibition in leukocytes diminishes inflammation via effects on integrins/cytoskeleton and protects the blood-brain barrier
Journal of Neuroinflammation (2016)
-
The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an IL-10-dependent fashion
Acta Neuropathologica (2016)
-
Poly(ADP-ribose) Polymerase-1 Inhibition in Brain Endothelium Protects the Blood—Brain Barrier under Physiologic and Neuroinflammatory Conditions
Journal of Cerebral Blood Flow & Metabolism (2015)