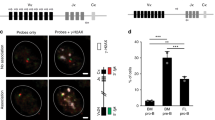
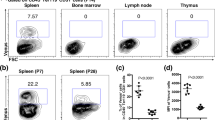
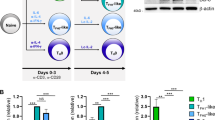
Abstract
The human body contains over 500 individual lymph nodes, yet the biology of their formation is poorly understood. Here we identify human lymphoid tissue–inducer cells (LTi cells) as lineage-negative RORC+ CD127+ cells with the functional ability to interact with mesenchymal cells through lymphotoxin and tumor necrosis factor. Human LTi cells were committed natural killer (NK) cell precursors that produced interleukin 17 (IL-17) and IL-22. In vitro, LTi cells gave rise to RORC+ CD127+ NK cells that retained the ability to produce IL-17 and IL-22. Postnatally, similar populations of LTi cell–like cells and RORC+ CD127+ NK cells were present in tonsils, and both secreted IL-17 and IL-22 but no interferon-γ. Our data indicate that lymph node organogenesis is controlled by an NK cell precursor population with adaptive immune features and demonstrate a previously unappreciated link between the innate and adaptive immune systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Drayton, D.L., Liao, S., Mounzer, R.H. & Ruddle, N.H. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7, 344–353 (2006).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Mebius, R.E. et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol. 166, 6593–6601 (2001).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Kurebayashi, S. et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. USA 97, 10132–10137 (2000).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Boos, M.D., Yokota, Y., Eberl, G. & Kee, B.L. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130 (2007).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Kyriazis, A.A. & Esterly, J.R. Development of lymphoid tissues in the human embryo and early fetus. Arch. Pathol. 90, 348–353 (1970).
Markgraf, R., von Gaudecker, B. & Muller-Hermelink, H.K. The development of the human lymph node. Cell Tissue Res. 225, 387–413 (1982).
Spencer, J., Finn, T. & Isaacson, P.G. Human Peyer's patches: an immunohistochemical study. Gut 27, 405–410 (1986).
Spencer, J., MacDonald, T.T., Finn, T. & Isaacson, P.G. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin. Exp. Immunol. 64, 536–543 (1986).
Mebius, R.E., Rennert, P. & Weissman, I.L. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Facchetti, F., Blanzuoli, L., Ungari, M., Alebardi, O. & Vermi, W. Lymph node pathology in primary combined immunodeficiency diseases. Springer Semin. Immunopathol. 19, 459–478 (1998).
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 2, 223–238 (1995).
Park, S.Y. et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3, 771–782 (1995).
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17, 823–833 (2002).
Pahal, G.S., Jauniaux, E., Kinnon, C., Thrasher, A.J. & Rodeck, C.H. Normal development of human fetal hematopoiesis between eight and seventeen weeks' gestation. Am. J. Obstet. Gynecol. 183, 1029–1034 (2000).
Cupedo, T. et al. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J. Immunol. 173, 4889–4896 (2004).
Hirose, T., Smith, R.J. & Jetten, A.M. ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem. Biophys. Res. Commun. 205, 1976–1983 (1994).
Anker, P.S. et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88, 845–52 (2003).
Dontje, W. et al. Delta-like1-induced Notch1 signaling regulates the human plasmacytoid dendritic cell versus T-cell lineage decision through control of GATA-3 and Spi-B. Blood 107, 2446–2452 (2006).
Schotte, R. et al. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood 101, 1015–1023 (2003).
Freud, A.G. et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22, 295–304 (2005).
Freud, A.G. & Caligiuri, M.A. Human natural killer cell development. Immunol. Rev. 214, 56–72 (2006).
Freud, A.G. et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J. Exp. Med. 203, 1033–1043 (2006).
Nishikawa, S.I., Hashi, H., Honda, K., Fraser, S. & Yoshida, H. Inflammation, a prototype for organogenesis of the lymphopoietic/hematopoietic system. Curr. Opin. Immunol. 12, 342–345 (2000).
Jones, C.E. & Chan, K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-α, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 26, 748–753 (2002).
McAllister, F. et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 175, 404–412 (2005).
Ouyang, W., Kolls, J.K. & Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 (2008).
Boniface, K. et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 174, 3695–3702 (2005).
Sa, S.M. et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J. Immunol. 178, 2229–2240 (2007).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Scandella, E. et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 9, 667–675 (2008).
Coles, M.C. et al. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc. Natl. Acad. Sci. USA 103, 13457–13462 (2006).
Aloisi, F. & Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6, 205–217 (2006).
Armengol, M.P. et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active Intrathyroidal germinal centers. Am. J. Pathol. 159, 861–873 (2001).
Weninger, W. et al. Naive T cell Recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 170, 4638–4648 (2003).
Luci, C. et al. Differential roles of the transcription factor RORγt in the development of NKp46+ lymphoid tissue–inducer–like cells and NKp46− natural killer cells in gut and skin. Nat. Immunol. advance online publication, 10.1038/ni1681 (23 November 2008).
Sanos, S.L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat. Immunol. advance online publication, 10.1038/ni1684 (23 November 2008).
Boehm, T. & Bleul, C.C. The evolutionary history of lymphoid organs. Nat. Immunol. 8, 131–135 (2007).
de Rie, M.A., Zeijlemaker, W.P. & von dem Borne, A.E. Inhibition, by vinca alkaloids and colchicine, of antigenic modulation induced by anti-CD19 monoclonal antibodies. Leuk. Res. 12, 135–141 (1988).
Acknowledgements
We thank the staff of the Centers for Contraception, Sexual Health and Abortion in Leiden and Rotterdam and the Bloemenhove clinic in Heemstede for collection of fetal tissues; Y. Simons-Oosterhuis for help with quantitative PCR; M. Kuijf for acquiring tonsil tissue; and J. Samsom, R. Mebius, L. Lanier, L. Meyaard and members of the Hematology department of the Erasmus University Medical Center for critical reading of the manuscript. Supported by The Netherlands Organization for Scientific Research (VENI grant 916.66.018 to T.C.).
Author information
Authors and Affiliations
Contributions
T.C. and H.S. conceived the work; T.C. designed and did experiments for Figures 1, 2, 3, 4, 5, 6, 7, analyzed results and wrote the manuscript; N.K.C. designed, did and analyzed experiments for Figure 8; N.P. did experiments for Figures 1, 2, 3, 4, 5, 6, 7; E.J.R. assisted in flow cytometry and experimental procedures; K.W. isolated fetal organs; J.L.G. constructed the LTβR-Ig fusion protein; W.E.F. provided mesenchymal stem cells; J.J.C. assisted with the design of the experiments and interpretation of the data; H.S. designed experiments, analyzed data and cowrote the manuscript; and all authors read and provided comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.K.C., J.L.G. and H.S. are employees of Genentech, a company that develops and markets therapeutic drugs.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 249 kb)
Rights and permissions
About this article
Cite this article
Cupedo, T., Crellin, N., Papazian, N. et al. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+ CD127+ natural killer–like cells. Nat Immunol 10, 66–74 (2009). https://doi.org/10.1038/ni.1668
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1668
This article is cited by
-
Reciprocal costimulatory molecules control the activation of mucosal type 3 innate lymphoid cells during engagement with B cells
Cellular & Molecular Immunology (2023)
-
The roles of tertiary lymphoid structures in chronic diseases
Nature Reviews Nephrology (2023)
-
Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease
Journal of Neural Transmission (2023)
-
Notch, RORC and IL-23 signals cooperate to promote multi-lineage human innate lymphoid cell differentiation
Nature Communications (2022)
-
The unique role of innate lymphoid cells in cancer and the hepatic microenvironment
Cellular & Molecular Immunology (2022)