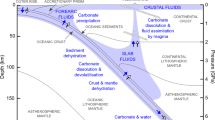
Abstract
Supercritical aqueous fluids link subducting plates and the return of carbon to Earth’s surface in the deep carbon cycle1,2. The amount of carbon in the fluids and the identities of the dissolved carbon species are not known, which leaves the deep carbon budget poorly constrained3. Traditional models4,5, which assume that carbon exists in deep fluids as dissolved gas molecules, cannot predict the solubility and ionic speciation of carbon in its silicate rock environment. Recent advances enable these limitations to be overcome when evaluating the deep carbon cycle6,7,8. Here we use the Deep Earth Water theoretical model7 to calculate carbon speciation and solubility in fluids under upper mantle conditions. We find that fluids in equilibrium with mantle peridotite minerals generally contain carbon in a dissolved gas molecule form. However, fluids in equilibrium with diamonds and eclogitic minerals in the subducting slab contain abundant dissolved organic and inorganic ionic carbon species. The high concentrations of dissolved carbon species provide a mechanism to transport large amounts of carbon out of the subduction zone, where the ionic carbon species may influence the oxidation state of the mantle wedge. Our results also identify novel mechanisms that can lead to diamond formation and the variability of carbon isotopic composition via precipitation of the dissolved organic carbon species in the subduction-zone fluids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ague, J. J. & Nicolescu, S. Carbon dioxide released from subduction zones by fluid-mediated reactions. Nature Geosci. 7, 355–360 (2014).
Frezzotti, M. L., Selverstone, J., Sharp, Z. D. & Compagnoni, R. Carbonate dissolution during subduction revealed by diamond-bearing rocks from the Alps. Nature Geosci. 4, 703–706 (2011).
Manning, C. E. Geochemistry: A piece of the deep carbon puzzle. Nature Geosci. 7, 333–334 (2014).
Connolly, J. A. D. Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet. Sci. Lett. 236, 524–541 (2005).
Zhang, C. & Duan, Z. A model for C–O–H fluid in the Earth’s mantle. Geochim. Cosmochim. Acta 73, 2089–2102 (2009).
Facq, S., Daniel, I. & Sverjensky, D. A. In situ Raman study and thermodynamic model of aqueous carbonate speciation in equilibrium with aragonite under subduction zone conditions. Geochim. Cosmochim. Acta 132, 375–390 (2014).
Sverjensky, D. A., Harrison, B. & Azzolini, D. Water in the deep Earth: The dielectric constant and the solubilities of quartz and corundum to 60 kb and 1,200 °C. Geochim. Cosmochim. Acta 129, 125–145 (2014).
Manning, C. E. Thermodynamic modeling of fluid–rock interaction at mid-crustal to upper-mantle conditions. Rev. Mineral. Geochem. 76, 135–164 (2013).
Dasgupta, R. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochem. 75, 183–229 (2013).
Evans, K. A. The redox budget of subduction zones. Earth Sci. Rev. 113, 11–32 (2012).
Frost, D. J. & McCammon, C. A. The redox state of Earth’s mantle. Annu. Rev. Earth Planet. Sci. 36, 389–420 (2008).
Pan, D., Spanu, L., Harrison, B., Sverjensky, D. A. & Galli, G. The dielectric constant of water under extreme conditions and transport of carbonates in the deep earth. Proc. Natl Acad. Sci. USA 110, 6646–6650 (2013).
Tomlinson, E. L., Jones, A. P. & Harris, J. W. Co-existing fluid and silicate inclusions in mantle diamond. Earth Planet. Sci. Lett. 250, 581–595 (2006).
Caciagli, N. & Manning, C. The solubility of calcite in water at 6–16 kbar and 500–800°C. Contrib. Mineral. Petrol. 146, 275–285 (2003).
Manning, C. E., Shock, E. L. & Sverjensky, D. A. The chemistry of carbon in aqueous fluids at crustal and upper-mantle conditions: Experimental and theoretical constraints. Rev. Mineral. Geochem. 75, 108–148 (2013).
Shirey, S. B. et al. Diamonds and the geology of mantle carbon. Rev. Mineral. Geochem. 75, 355–421 (2013).
Hammouda, T. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 214, 357–368 (2003).
Hacker, B. R. H2O subduction beyond arcs. Geochem. Geophys. Geosys. 9, Q03001 (2008).
Stagno, V., Ojwang, D. O., McCammon, C. A. & Frost, D. J. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 493, 84–88 (2013).
Stagno, V., Frost, D. J. & McCammon, C. A. AGU Fall Meeting Abstracts Vol. 1, Abstract no. DI21A-2060 (American Geophysical Union, 2011).
Frezzotti, M-L., Huizenga, J-M., Compagnoni, R. & Selverstone, J. Diamond formation by carbon saturation in C–O–H fluids during cold subduction of oceanic lithosphere. Geochim. Cosmochim. Acta 143, 68–86 (2013).
Van Keken, P. E., Hacker, B. R., Syracuse, E. M. & Abers, G. A. Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide. J. Geophys. Res. 116, B01401 (2011).
Stagno, V. & Frost, D. J. Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth Planet. Sci. Lett. 300, 72–84 (2010).
Hermann, J., Zheng, Y-F. & Rubatto, D. Deep fluids in subducted continental crust. Elements 9, 281–287 (2013).
Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposits. Econ. Geol. 67, 551–578 (1972).
Shock, E. L., Oelkers, E. H., Johnson, J. W., Sverjensky, D. A. & Helgeson, H. C. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Effective electrostatic radii to 1000 °C and 5 kb. Faraday Soc. Trans. 88, 803–826 (1992).
Bowers, T. S., Jackson, K. J. & Helgeson, H. C. Equilibrium Activity Diagrams (Springer, 1984).
Berman, R. G. Internally-consistent thermodynamic data for minerals in the system Na2O–K2O–CaO–MgO–FeO–Fe2O3–Al2O3–SiO2–TiO2–H2O–CO2 . J. Petrol. 29, 445–522 (1988).
Holland, T. J. B. & Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 29, 333–383 (2011).
Sverjensky, D. A., Hemley, J. J. & D’Angelo, W. M. Thermodynamic assessment of hydrothermal alkali feldspar–mica–aluminosilicate equilibria. Geochim. Cosmochim. Acta 55, 989–1004 (1991).
Acknowledgements
This research was supported by the WDC Research Fund (V.S.), a Johns Hopkins Graduate Fellowship (F.H.), grants from the Sloan Foundation through the Deep Carbon Observatory (Reservoirs and Fluxes and Extreme Physics and Chemistry programmes to D.A.S.) and grant DOE DE-FG-02-96ER-14616 (D.A.S.). We are also grateful for the help and support of the Johns Hopkins University and the Geophysical Laboratory of the Carnegie Institution of Washington. We wish to acknowledge reviews of the manuscript by R. E. Cohen, R. M. Hazen and C. M. Schiffries, as well as helpful discussions with I. Daniel, Y. Fei, M. S. Ghiorso, R. J. Hemley, S. Lobanov, C. E. Manning, S. Mikhail, B. O. Mysen and E. L. Shock.
Author information
Authors and Affiliations
Contributions
All authors contributed to the calculations and the writing of the manuscript. The activity diagrams were calculated by F.H., the aqueous speciation and solubility modelling was carried out by D.A.S. The selection of systems of interest and oxidation states in the mantle were guided and calculated by V.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 16990 kb)
Rights and permissions
About this article
Cite this article
Sverjensky, D., Stagno, V. & Huang, F. Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nature Geosci 7, 909–913 (2014). https://doi.org/10.1038/ngeo2291
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2291
This article is cited by
-
Early release of H2O during subduction of carbonated ultramafic lithologies
Contributions to Mineralogy and Petrology (2023)
-
Nanoconfinement facilitates reactions of carbon dioxide in supercritical water
Nature Communications (2022)
-
Decoupling of inorganic and organic carbon during slab mantle devolatilisation
Nature Communications (2022)
-
Deep carbon cycle constrained by carbonate solubility
Nature Communications (2021)
-
Insights on the deep carbon cycle from the electrical conductivity of carbon-bearing aqueous fluids
Scientific Reports (2021)