Abstract
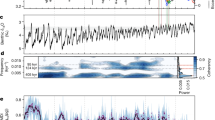
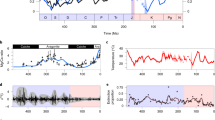
Coccolithophores—single-celled calcifying phytoplankton—represent an essential footing to marine ecosystems, yet their sensitivity to environmental change, and in particular increases in atmospheric CO2, is poorly understood1. During the Palaeocene–Eocene Thermal Maximum (PETM), about 56 million years ago, atmospheric CO2 concentrations rose rapidly and the oceans acidified2,3, making this an ideal time interval to examine coccolithophore responses to environmental change. Here we compare the results of experiments on modern coccolithophore species with exceptional fossil coccosphere records of the PETM, providing a cellular-level perspective. In modern taxa, we find that during the exponential growth phase of rapid cell division, small cells with few coccoliths are produced, whereas larger cells with more coccoliths are produced during slowed cell division. Applying these diagnostic features to the PETM fossil record, we find that the dominant species exhibited different growth responses to the environmental forcing. Toweius pertusus shows geometry indicative of rapid cell division. In contrast, we suggest that cells of Coccolithus pelagicus grew more slowly during the period of environmental change. In the modern ocean, Emiliania huxleyi, which is closely related to the extinct T. pertusus, is prolific and widespread, whereas C. pelagicus is more limited in range and abundance. We argue that these different responses to environmental change were critical to the post-PETM evolutionary success of the descendants of these taxa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ridgwell, et al. From laboratory manipulations to Earth system models: Scaling calcification impacts of ocean acidification. Biogeosciences 6, 2611–2623 (2009).
Sluijs, A., Bowen, G. J., Brinkhuis, H., Lourens, L. J. & Thomas, E. in Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies (eds Williams, M., Haywood, A. M., Gregory, F. J. & Schmidt, D. N.) 323–349 (The Micropaleontological Society, Special Publications, The Geological Society, 2007).
Zachos, J. C., Dickens, G. R. & Zeebe, R. E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283 (2008).
Bown, P. R. et al. A Paleogene calcareous microfossil Konservat-Lagerstätte from the Kilwa Group of coastal Tanzania. GSA Bull. 120, 3–12 (2008).
Hönisch, B. et al. The geological record of ocean acidification. Science 335, 1058–1063 (2012).
Bralower, T. J. Evidence of surface water oligotrophy during the PETM: Nannofossil assemblage data from Ocean Drilling Program Site 690, Maud Rise, Weddell Sea. Paleoceanography 17, 1023 (2002).
Gibbs, S. J., Bralower, T. J., Bown, P. R., Zachos, J. C. & Bybell, L. Shelf and open-ocean calcareous phytoplankton assemblages across the Paleocene–Eocene Thermal Maximum: Implications for global productivity gradients. Geology 34, 233–236 (2006).
Bown, P. & Pearson, P. Calcareous plankton evolution and the Paleocene/Eocene thermal maximum event: New evidence from Tanzania. Mar. Micropaleontol. 71, 60–70 (2009).
Gibbs, S. J., Bown, P. R., Sessa, J., Bralower, T. J. & Wilson, P. A. Nannoplankton extinction and origination rates across the Paleocene–Eocene Thermal Maximum. Science 314, 1770–1773 (2006).
Young, J. R. et al. A guide to extant coccolithophore taxonomy. J. Nannoplankton Res., Special Publication 1, 1–125 (2003).
Bown, P. R., Lees, J. A. & Young, J. R. in Coccolithophores—From Molecular Processes to Global Impacts (eds Thierstein, H. & Young, J. R.) 481–508 (Springer, 2004).
Gallagher, L. in Nannofossils and Their Application (eds Crux, J. & Heck, S. E. van) 41–75 (Ellis Horwood, 1989).
Balch, W. M., Kilpatrick, K., Holligan, P. M. & Cucci, T. Coccolith production and detachment by Emiliania huxleyi (Prymnesiophyceae). J. Phycol. 29, 566–575 (1993).
Fritz, J. J. Carbon fixation and coccolith detachment in the coccolithophore Emiliania huxleyi in nitrate-limited cyclostats. Mar. Biol. 133, 509–518 (1999).
Müller, M. N., Antia, A. N. & LaRoche, J. Influence of cell cycle phase on calcification in the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 53, 506–512 (2008).
Paache, E. Roles of nitrogen and phosphorus in coccolith formation in Emiliania huxleyi (Prymnesiophyceae). Eur. J. Phycol. 33, 33–42 (1998).
Riegman, R., Stole, W., Noordeloos, A. A. M. & Slezak, D. Nutrient uptake and alkaline phosphatase (EC 3:1:3:1) activity of Emiliania huxleyi (Prymnesiophyceae) during growth under N and P limitation in continuous cultures. J. Phycol. 36, 87–96 (2000).
Langer, G. et al. Calcium isotope fractionation during coccolith formation in Emiliania huxleyi: Independence of growth and calcification rate. Geochem. Geophys. Geosyst. 8, Q05007 (2007).
Langer, G. et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst. 7, Q09006 (2006).
Langer, G., Nehrke, G., Probert, I., Ly, J. & Ziveri, P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst. 6, 2637–2646 (2009).
John, C. M. et al. North American continental margin records of the Paleocene–Eocene thermal maximum: Implications for global carbon and hydrological cycling. Paleoceanography 23, PA2217 (2008).
Zachos, J. C. et al. Extreme warming of the mid-latitude coastal ocean during the Paleocene–Eocene Thermal Maximum: Inferences from TEX86 and isotope data. Geology 34, 737–740 (2006).
Sato, T., Yuguchi, S., Takayama, T. & Kameo, K. Drastic change in the geographical distribution of the cold-water nannofossil Coccolithus pelagicus (Wallich) Schiller at 2.74 Ma in the late Pliocene, with special reference to glaciation in the Arctic Ocean. Mar. Micropaleontol. 52, 181–193.
Ziveri, P., Baumann, K. H., Böckel, B., Bollmann, J. & Young, J. R. in Coccolithophores—From Molecular Processes to Global Impacts (eds Thierstein, H. & Young, J. R.) 403–428 (Springer, 2004).
Miller, K. G. et al. in Proceedings of the Ocean Drilling Program, Scientific Results 174AX (eds Miller, K. G. et al.) 5–43 (Ocean Drilling Program, 1998).
Nicholas, C. J. et al. Stratigraphy and sedimentology of the Upper Cretaceous to Paleogene Kilwa Group, southern coastal Tanzania. J. Afr. Earth Sci. 45, 431–466 (2006).
Montadert, L. et al. Initial Reports of the Deep Sea Drilling Project Vol. 48 (US Government Printing Office, 1979).
Lampitt, R. et al. Particulate export from the euphotic zone: Estimates using a novel drifting sediment trap, 234 Th and new production. Deep-Sea Res. I 55, 1483–1502 (2008).
Henderiks, J. Coccolithophore size rules—reconstructing ancient cell geometry and cellular calcite quota from fossil coccoliths. Mar. Micropaleontol. 67, 143–154 (2008).
Nunes, F. & Norris, R. D. Abrupt reversal in ocean overturning during the Palaeocene/Eocene warm period. Nature 439, 60–63 (2006).
Acknowledgements
The authors would like to acknowledge research support from the Royal Society through a University Research Fellowship to S.J.G., from the Natural Environment Research Council through a Post-doctoral Fellowship to A.J.P. (NE/F015054/1) and studentships to S.A.O., J.H. and C.J.D. and a research experience placement for C.N., and an added value grant from the UK Ocean Acidification programme (which includes funding from NERC, DECC and DEFRA). Part of this collaborative research between modern and palaeo research arose as a result of the UKOA project and we thank the consortium group principal investigators (P. Pearson, G. Foster and T. Tyrell) and also the UKOA project manager P. Williamson. We would also like to thank I. Probert for advice on coccolithophore culturing, R. Pearce for assistance with SEM imaging, and M. Coupel, J. Hurst, E. Maher, R. Lampitt, S. Ward and C. Marsay for assistance in collection and analysis of water and sediment trap samples from the Iceland Basin in 2010. Thanks also to D. Iglesias-Rodriguez and C. Newe for invaluable discussions, E. J. Rohling for manuscript editing, and A. Westmacott and Olympus Keymed for hardware and software support.
Author information
Authors and Affiliations
Contributions
S.J.G., P.R.B. and A.J.P. conceived and designed the research and experiments, with advice from J.R.Y. S.J.G. performed all biometric analyses; A.J.P., J.H. and C.J.D. performed the culture experiments, collected daily cell counts and imaged samples under SEM. P.R.B. performed fossil SEM imaging. H.L.J., G.J.T., S.A.O. and C.N. provided further fossil biometric data. S.J.G., A.J.P., P.R.B. and J.R.Y. interpreted findings and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 804 kb)
Rights and permissions
About this article
Cite this article
Gibbs, S., Poulton, A., Bown, P. et al. Species-specific growth response of coccolithophores to Palaeocene–Eocene environmental change. Nature Geosci 6, 218–222 (2013). https://doi.org/10.1038/ngeo1719
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1719
This article is cited by
-
Eocene emergence of highly calcifying coccolithophores despite declining atmospheric CO2
Nature Geoscience (2022)
-
Abrupt conclusion of the late Miocene-early Pliocene biogenic bloom at 4.6-4.4 Ma
Nature Communications (2022)
-
High resolution spatial analyses of trace elements in coccoliths reveal new insights into element incorporation in coccolithophore calcite
Scientific Reports (2020)
-
A new method for isolating and analysing coccospheres within sediment
Scientific Reports (2020)
-
X-ray nanotomography of coccolithophores reveals that coccolith mass and segment number correlate with grid size
Nature Communications (2019)