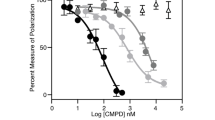
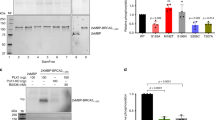
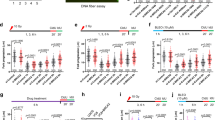
Abstract
The breast cancer tumor-suppressor gene, BRCA1, encodes a protein with a BRCT domain—a motif that is found in many proteins that are implicated in DNA damage response and in genome stability1. Phosphorylation of BRCA1 by the DNA damage-response proteins ATM, ATR and hCds1/Chk2 changes in response to DNA damage and at replication-block checkpoints2,3,4,5. Although cells that lack BRCA1 have an abnormal response to DNA damage, the exact role of BRCA1 in this process has remained unclear. Here we show that BRCA1 is essential for activating the Chk1 kinase that regulates DNA damage–induced G2/M arrest. Thus, BRCA1 controls the expression, phosphorylation and cellular localization of Cdc25C and Cdc2/cyclin B kinase—proteins that are crucial for the G2/M transition. We show that BRCA1 regulates the expression of both Wee1 kinase, an inhibitor of Cdc2/cyclin B kinase, and the 14-3-3 family of proteins that sequesters phosphorylated Cdc25C and Cdc2/cyclin B kinase in the cytoplasm6. We conclude that BRCA1 regulates key effectors that control the G2/M checkpoint and is therefore involved in regulating the onset of mitosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bork, P. et al. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 11, 68–76 (1997).
Cortez, D., Wang, Y., Qin, J. & Elledge, S.J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science 286, 1162–1166 (1999).
Tibbetts, R.S. et al. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14, 2989–3002 (2000).
Lee, J.S., Collins, K.M., Brown, A.L., Lee, C.H. & Chung, J.H. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404, 201–204 (2000).
Chen, J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 60, 5037–5039 (2000).
Lopez-Girona, A., Furnari, B., Mondesert, O. & Russell, P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397, 172–175 (1999).
Tomlinson, G.E. et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 58, 3237–3242 (1998).
Scully, R. et al. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol. Cell 4, 1093–1099 (1999).
Foray, N. et al. Γ-rays-induced death of human cells carrying mutations of BRCA1 or BRCA2. Oncogene 18, 7334–7342 (1999).
Xu, B., Kim, S. & Kastan, M.B. Involvement of BRCA1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21, 3445–3450 (2001).
Zhong, Q. et al. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 285, 747–750 (1999).
MacLachlan, T.K. et al. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J. Biol. Chem. 275, 2777–2785 (2000).
O'Connor, P.M. et al. G2 delay induced by nitrogen mustard in human cells affects cyclin A/Cdk2 and cyclin B1/Cdc2-kinase complexes differently. J. Biol. Chem. 268, 8298–8308 (1993).
Rhind, N., Furnari, B. & Russell, P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 11, 504–511 (1997).
Lee, M.S. et al. Cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol. Biol. Cell 3, 73–84 (1992).
Ogg, S., Gabrielli, B. & Piwnica-Worms, H. Purification of a serine kinase that associates with and phosphorylates human Cdc25C on serine 216. J. Biol. Chem. 269, 30461–30469 (1994).
Furnari, B., Rhind, N. & Russell, P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science 277, 1495–1497 (1997).
Sanchez, Y. et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–1501 (1997).
Peng, C.Y. et al. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501–1505 (1997).
Hermeking, H. et al. 14-3-3σ is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1, 3–11 (1997).
Matsuoka, S., Huang, M. & Elledge, S.J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893–1897 (1998).
Xu, X. et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3, 389–395 (1999).
Xu, X. et al. Genetic interactions between tumor suppressors BRCA1 and TP53 in apoptosis, cell cycle and tumorigenesis. Nature Genet. 28, 266–271 (2001).
van den Heuvel, S. & Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262, 2050–2054 (1993).
Yarden, R.I. & Brody, L.C. BRCA1 interacts with components of the histone deacetylase complex. Proc. Natl Acad. Sci. USA 96, 4983–4988 (1999).
Acknowledgements
We thank Y. Pommier for the GST–Cdc25C plasmid, C. Smythe for the Chk2 antibody, Y. Shiloh for AT cells, S. Anderson and M. Kirby for flow cytometry, A. Dutra for help with confocal microscopy, E. Sausville and the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis at the National Cancer Institute for providing UCN-01; and P. Liu and S. Danoff for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yarden, R., Pardo-Reoyo, S., Sgagias, M. et al. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet 30, 285–289 (2002). https://doi.org/10.1038/ng837
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng837
This article is cited by
-
Enhanced efficacy of combined fluzoparib and chidamide targeting in natural killer/T-cell lymphoma
Annals of Hematology (2023)
-
A synergetic effect of BARD1 mutations on tumorigenesis
Nature Communications (2021)
-
Single-cell transcriptome profiling of buffelgrass (Cenchrus ciliaris) eggs unveils apomictic parthenogenesis signatures
Scientific Reports (2021)
-
Testis transcriptome profiling identified lncRNAs involved in spermatogenic arrest of cattleyak
Functional & Integrative Genomics (2021)
-
Elucidating the cellular response of silver nanoparticles as a potential combinatorial agent for cisplatin chemotherapy
Journal of Nanobiotechnology (2020)