Abstract
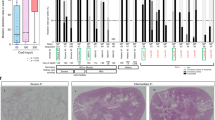
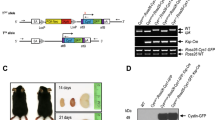
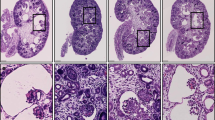
Autosomal recessive polycystic kidney disease (ARPKD) is characterized by dilation of collecting ducts and by biliary dysgenesis and is an important cause of renal- and liver-related morbidity and mortality. Genetic analysis of a rat with recessive polycystic kidney disease revealed an orthologous relationship between the rat locus and the ARPKD region in humans; a candidate gene was identified. A mutation was characterized in the rat and screening the 66 coding exons of the human ortholog (PKHD1) in 14 probands with ARPKD revealed 6 truncating and 12 missense mutations; 8 of the affected individuals were compound heterozygotes. The PKHD1 transcript, approximately 16 kb long, is expressed in adult and fetal kidney, liver and pancreas and is predicted to encode a large novel protein, fibrocystin, with multiple copies of a domain shared with plexins and transcription factors. Fibrocystin may be a receptor protein that acts in collecting-duct and biliary differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guay-Woodford, L.M. Autosomal recessive polycystic kidney disease: clinical and genetic profiles. in Polycystic Kidney Disease (eds Watson, M.L. & Torres, V.E.) 237–266 (Oxford University Press, New York, 1996).
Zerres, K., Rudnik-Schöneborn, S., Steinkamm, C., Becker, J. & Mücher, G. Autosomal recessive polycystic kidney disease. J. Mol. Med. 76, 303–309 (1998).
MacRae Dell, K.M. & Avner, E.D. Autosomal recessive polycystic kidney disease. in GeneReviews; Genetic Disease Online Reviews at GeneTests-GeneClinics (University of Washington, Seattle, 2001).
Zerres, K., Volpel, M.-C. & Wei, B.H. Cystic kidneys. Hum. Genet. 68, 104–135 (1984).
McDonald, R., Watkins, S.L. & Avner, E.D. Polycystic kidney disease. in Pediatric Nephrology (eds Barratt, T.M., Avner, E.D. & Harmon, W.E.) 459–480 (Lippincott, Williams and Wilkins, Baltimore, 1999).
Bernstein, J. & Slovis, T.L. Polycystic diseases of the kidney. in Pediatric Kidney Disease Vol. 2 (ed. Edelmann, C.M.) 1139–1157 (Little, Brown, Boston, 1992).
Zerres, K. et al. Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): molecular genetics, clinical experience, and fetal morphology. Am. J. Med. Genet. 76, 137–144 (1998).
Roy, S., Dillon, M.J., Trompeter, R.S. & Barratt, T.M. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr. Nephrol. 11, 302–306 (1997).
Kaplan, B.S., Fay, J., Shah, V., Dillon, M.J. & Barratt, T.M. Autosomal recessive polycystic kidney disease. Pediatr. Nephrol. 3, 43–49 (1989).
Kaariainen, H., Koskimies, O. & Norio, R. Dominant and recessive polycystic kidney disease in children: evaluation of clinical features and laboratory data. Pediatr. Nephrol. 2, 296–302 (1988).
Osathanondh, V. & Potter, E.L. Pathogenesis of polycystic kidneys. Type 1 due to hyperplasia of interstitial portions of the collecting tubules. Archives of Pathology 77, 466–473 (1964).
Blyth, H. & Ockenden, B.G. Polycystic disease of kidneys and liver presenting in childhood. J. Med. Genet. 8, 257–284 (1971).
Cole, B.R., Conley, S.B. & Stapleton, F.B. Polycystic kidney disease in the first year of life. J. Pediatr. 111, 693–699 (1987).
Zerres, K. et al. Autosomal recessive polycystic kidney disease in 115 children: clinical presentation, course and influence of gender. Acta. Paediatr. 85, 437–445 (1996).
Jorgensen, M.J. The ductal plate malformation: a study of the intrahepatic bile duct lesion in infantile polycystic disease and congenital hepatic fibrosis. Acta Pathol. Microbiol. Scand. Suppl. 257, 1–87 (1977).
Desmet, V.J. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology 16, 1069–1083 (1992).
Calvet, J.P. Polycystic kidney disease: primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int. 43, 101–108 (1993).
Kaplan, B.S. et al. Variable expression of autosomal recessive polycystic kidney disease and congenital hepatic fibrosis within a family. Am. J. Med. Genet. 29, 639–647 (1988).
Deget, F., Rudnik-Schoneborn, S. & Zerres, K. Course of autosomal recessive polycystic kidney disease (ARPKD) in siblings: a clinical comparison of 20 sibships. Clin. Genet. 47, 248–253 (1995).
Zerres, K. et al. Mapping of the gene for autosomal recessive polycystic kidney disease (ARPKD) to chromosome 6p21-cen. Nature Genet. 7, 429–432 (1994).
Guay-Woodford, L.M. et al. The severe perinatal form of autosomal recessive polycystic kidney disease maps to chromosome 6p21.1-p12: implications for genetic counseling. Am. J. Hum. Genet. 56, 1101–1107 (1995).
Lens, X.M. et al. An integrated genetic and physical map of the autosomal recessive polycystic kidney disease region. Genomics 41, 463–466 (1997).
Mücher, G. et al. Fine mapping of the autosomal recessive polycystic kidney disease locus (PKHD1) and the genes MUT, RDS, CSNK2β, and GSTA1 at 6p21.2-p12. Genomics 48, 40–45 (1998).
Park, J.H. et al. A 1-Mb BAC/PAC-based physical map of the autosomal recessive polycystic kidney disease gene (PKHD1) region on chromosome 6. Genomics 57, 249–255 (1999).
Onuchic, L.F. et al. Genomic organization of the KIAA0057 gene that encodes a TRAM-like protein and its exclusion as a polycystic kidney and hepatic disease 1 (PKHD1) candidate gene. Mamm. Genome 10, 1175–1178 (1999).
Hofmann, Y. et al. Genomic structure of the gene for the human P1 protein (MCM3) and its exclusion as a candidate for autosomal recessive polycystic kidney disease. Eur. J. Hum. Genet. 8, 163–166 (2000).
McDonald, R.A. & Avner, E.D. Mouse models of polycystic kidney disease. in Polycystic Kidney Disease (eds Watson, M.L. & Torres, V.E.) 63–87 (Oxford University Press, Oxford, 1996).
Moyer, J.H. et al. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 263, 1329–1333 (1994).
Lager, D.J., Qian, Q., Bengal, R.J., Ishibashi, M. & Torres, V.E. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 59, 126–136 (2001).
Sanzen, T. et al. Polycystic kidney rat is a novel animal model of Caroli's disease associated with congenital hepatic fibrosis. Am. J. Pathol. 158, 1605–1612 (2001).
Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15, 8125–8148 (1987).
Rossetti, S. et al. A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int. (in press) (2002).
Xiao, W. & Oefner, P.J. Denaturing high-performance liquid chromatography: a review. Hum. Mutat. 17, 439–474 (2001).
MacKay, K. et al. Glomerular epithelial, mesangial, and endothelial cell lines from transgenic mice. Kidney Int. 33, 677–684 (1988).
Bork, P., Doerks, T., Springer, T.A. & Snel, B. Domains in plexins: links to integrins and transcription factors. Trends Biochem. Sci. 24, 261–263 (1999).
Park, M. et al. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl Acad. Sci. USA 84, 6379–6383 (1987).
Tamagnone, L. et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80 (1999).
Wang, M.H. et al. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science 266, 117–119 (1994).
Scott, D.A. et al. Refining the DFNB7-DFNB11 deafness locus using intragenic polymorphisms in a novel gene, TMEM2. Gene 246, 265–274 (2000).
Pearson, R.B. & Kemp, B.E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 200, 62–81 (1991).
Blickman, J.G., Bramson, R.T. & Herrin, J.T. Autosomal recessive polycystic kidney disease: long-term sonographic findings in patients surviving the neonatal period. Am. J. Roentgenol. 164, 1247–1250 (1995).
Uhari, M. & Herva, R. Polycystic kidney disease of perinatal type. Acta Paediatr. Scand. 68, 443–444 (1979).
Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987).
Yeh, R.F., Lim, L.P. & Burge, C.B. Computational inference of homologous gene structures in the human genome. Genome Res. 11, 803–816 (2001).
Harris, P.C. et al. Rapid genetic analysis of families with polycystic kidney disease by means of a microsatellite marker. Lancet 338, 1484–1487 (1991).
Bateman, A. et al. The Pfam protein families database. Nucleic Acids Res. 28, 263–266 (2000).
Nielsen, H., Engelbrecht, J., Brunak, S. & von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10, 1–6 (1997).
Sonnhammer, E.L.L., von Heijne, G. & Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. in Proceedings of Sixth International Conference on Intelligent Systems for Molecular Biology (eds Glasgow, J., Littlejohn, T., Major, F., Lathrop, R., Sankoff, D. & Sensen, C.) 175–182 (AAAI, Menlo Park, 1998).
Claros, M.G. & von Heijne, G. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10, 685–686 (1994).
Rost, B., Fariselli, P. & Casadio, R. Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci. 5, 1704–1718 (1996).
Acknowledgements
We thank the patients and their families for taking part in the study, M. El Youssef, J. Gloor, E.N. Haugen, P.S. Kamath and B.Z. Morgenstern for referring patients, and P. Fraught, M. de Andrade and E.J. Atkinson for assistance. Charles River Japan are thanked for the gift of the breeding pairs of PCK rats. The work was supported by grants from the National Institutes of Health, the PKD Foundation (to S.R. and M.H.), the Mayo Graduate School and Foundation, Mayo Cancer Center and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ward, C., Hogan, M., Rossetti, S. et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30, 259–269 (2002). https://doi.org/10.1038/ng833
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng833
This article is cited by
-
Adult presentations of variable kidney and liver phenotypes secondary to biallelic PKHD1 pathogenic variants
Journal of Rare Diseases (2023)
-
Genetics, pathobiology and therapeutic opportunities of polycystic liver disease
Nature Reviews Gastroenterology & Hepatology (2022)
-
Generation of heterozygous PKD1 mutant pigs exhibiting early-onset renal cyst formation
Laboratory Investigation (2022)
-
Evaluation of galectin-3 and intestinal fatty acid binding protein as serum biomarkers in autosomal recessive polycystic kidney disease
Journal of Nephrology (2022)
-
Clinical features of autosomal recessive polycystic kidney disease in the Japanese population and analysis of splicing in PKHD1 gene for determination of phenotypes
Clinical and Experimental Nephrology (2022)