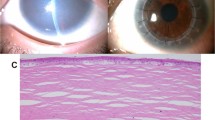
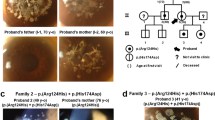
Abstract
Congenital hereditary endothelial dystrophy (CHED) is a heritable, bilateral corneal dystrophy characterized by corneal opacification and nystagmus. We describe seven different mutations in the SLC4A11 gene in ten families with autosomal recessive CHED. Mutations in SLC4A11, which encodes a membrane-bound sodium-borate cotransporter, cause loss of function of the protein either by blocking its membrane targeting or nonsense-mediated decay.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Accession codes
References
Chan, C.C., Green, W.R., Barraquer, J., Barraquer-Somers, E. & de la Cruz, Z.C. Cornea 1, 155–172 (1982).
Kirkness, C.M., McCarthy, A., Rice, N.S., Garner, A. & Steele, A.D. Br. J. Ophthalmol. 71, 130–144 (1987).
Toma, N.M. et al. Hum. Mol. Genet. 4, 2395–2398 (1995).
Hand, C.K. et al. Genomics 61, 1–4 (1999).
Mohamed, M.D. et al. Br. J. Ophthalmol. 85, 758–759 (2001).
Parker, M.D., Ourmozdi, E.P. & Tanner, M.J. Biochem. Biophys. Res. Commun. 282, 1103–1109 (2001).
Alper, S.L., Darman, R.B., Chernova, M.N. & Dahl, N.K. J. Nephrol. (Suppl.) 5, S41–S53 (2002).
Romero, M.F., Fulton, C.M. & Boron, W.F. Pflugers Arch. 447, 495–509 (2004).
Gottsch, J.D. et al. Invest. Ophthalmol. Vis. Sci. 44, 594–599 (2003).
Wilusz, C.J., Wormington, M. & Peltz, S.W. Nat. Rev. Mol. Cell Biol. 2, 237–246 (2001).
Park, M., Li, Q., Shcheynikov, N., Zeng, W. & Muallem, S. Mol. Cell 16, 331–341 (2004).
Acknowledgements
We thank the affected individuals and their families for participating in this study. This work was supported by a grant from the National Medical Research Council of Singapore (NMRC 0940/2005) and the Singapore Eye Research Institute. J.R.C. and P.M. are recipients of scientist and fellowship awards from the Alberta Heritage Foundation for Medical Research. Research in the laboratory of J.R.C. is funded by the Canadian Institutes of Health Research. We thank A. Liu and A. Barathi for assistance with dissection of human and mouse cornea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
CHED2 pedigree from Myanmar and cosegregation analysis of the R755Q mutation. (PDF 107 kb)
Supplementary Fig. 2
Expression of SLC4A11. (PDF 88 kb)
Supplementary Fig. 3
Physical map of CHED2 locus and SLC4A11 mutations. (PDF 285 kb)
Supplementary Fig. 4
Allele sharing between CHED2 families with common mutations G464D and R755Q. (PDF 37 kb)
Supplementary Fig. 5
Conservation of mutated residues of BTR1 as shown by multiple sequence alignment of human BTR1 with homologs and representative members of the human SLC4 family. (PDF 37 kb)
Supplementary Fig. 6
Immunoblot for the BTR1 wild-type (WT) mutants. (PDF 149 kb)
Rights and permissions
About this article
Cite this article
Vithana, E., Morgan, P., Sundaresan, P. et al. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38, 755–757 (2006). https://doi.org/10.1038/ng1824
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1824
This article is cited by
-
Boron-Containing Compounds for Prevention, Diagnosis, and Treatment of Human Metabolic Disorders
Biological Trace Element Research (2023)
-
Identification and in silico analysis of a spectrum of SLC4A11 variations in Indian familial and sporadic cases of congenital hereditary endothelial dystrophy
Orphanet Journal of Rare Diseases (2022)
-
Novel mutation in the TGFBI gene in a Moroccan family with atypical corneal dystrophy: a case report
BMC Medical Genomics (2021)
-
Altered gene expression in slc4a11−/− mouse cornea highlights SLC4A11 roles
Scientific Reports (2021)
-
Harboyan syndrome: novel SLC4A11 mutation, clinical manifestations, and outcome of corneal transplantation
Journal of Human Genetics (2021)