Abstract
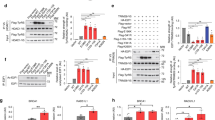
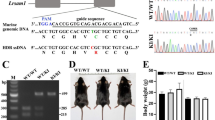
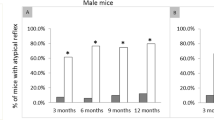
Charcot-Marie-Tooth (CMT) neuropathies are common disorders of the peripheral nervous system caused by demyelination or axonal degeneration, or a combination of both features. We previously assigned the locus for autosomal dominant intermediate CMT neuropathy type C (DI-CMTC) to chromosome 1p34-p35. Here we identify two heterozygous missense mutations (G41R and E196K) and one de novo deletion (153–156delVKQV) in tyrosyl-tRNA synthetase (YARS) in three unrelated families affected with DI-CMTC. Biochemical experiments and genetic complementation in yeast show partial loss of aminoacylation activity of the mutant proteins, and mutations in YARS, or in its yeast ortholog TYS1, reduce yeast growth. YARS localizes to axonal termini in differentiating primary motor neuron and neuroblastoma cultures. This specific distribution is significantly reduced in cells expressing mutant YARS proteins. YARS is the second aminoacyl-tRNA synthetase found to be involved in CMT, thereby linking protein-synthesizing complexes with neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Verhoeven, K. et al. Localization of the gene for the intermediate form of Charcot-Marie-Tooth to chromosome 10q24.1-q25.1. Am. J. Hum. Genet. 69, 889–894 (2001).
Kennerson, M.L. et al. Dominant intermediate Charcot-Marie-Tooth neuropathy maps to chromosome 19p12-p13.2. Am. J. Hum. Genet. 69, 883–888 (2001).
Jordanova, A. et al. Dominant intermediate Charcot-Marie-Tooth type C maps to chromosome 1p34-p35. Am. J. Hum. Genet. 73, 1423–1430 (2003).
Zuchner, S. et al. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 37, 289–294 (2005).
Fersht, A.R. et al. Active site titration and aminoacyl adenylate binding stoichiometry of aminoacyl-tRNA synthetases. Biochemistry 14, 1–4 (1975).
Wakasugi, K. & Schimmel, P. Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. J. Biol. Chem. 274, 23155–23159 (1999).
Kleeman, T.A., Wei, D., Simpson, K.L. & First, E.A. Human tyrosyl-tRNA synthetase shares amino acid sequence homology with a putative cytokine. J. Biol. Chem. 272, 14420–14425 (1997).
Wakasugi, K. & Schimmel, P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 (1999).
Winter, G., Fersht, A.R., Wilkinson, A.J., Zoller, M. & Smith, M. Redesigning enzyme structure by site-directed mutagenesis: tyrosyl tRNA synthetase and ATP binding. Nature 299, 756–758 (1982).
Ohno, S., Yokogawa, T. & Nishikawa, K. Changing the amino acid specificity of yeast tyrosyl-tRNA synthetase by genetic engineering. J. Biochem. 130, 417–423 (2001).
Wilkinson, A.J., Fersht, A.R., Blow, D.M. & Winter, G. Site-directed mutagenesis as a probe of enzyme structure and catalysis: tyrosyl-tRNA synthetase cysteine-35 to glycine-35 mutation. Biochemistry 22, 3581–3586 (1983).
Nair, S. et al. Species-specific tRNA recognition in relation to tRNA synthetase contact residues. J. Mol. Biol. 269, 1–9 (1997).
Yang, X.L., Skene, R.J., McRee, D.E. & Schimmel, P. Crystal structure of a human aminoacyl-tRNA synthetase cytokine. Proc. Natl. Acad. Sci. USA 99, 15369–15374 (2002).
Wakasugi, K., Quinn, C.L., Tao, N. & Schimmel, P. Genetic code in evolution: switching species-specific aminoacylation with a peptide transplant. EMBO J. 17, 297–305 (1998).
Sudhof, T.C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 375, 645–653 (1995).
Quevillon, S., Robinson, J.C., Berthonneau, E., Siatecka, M. & Mirande, M. Macromolecular assemblage of aminoacyl-tRNA synthetases: identification of protein-protein interactions and characterization of a core protein. J. Mol. Biol. 285, 183–195 (1999).
Yang, X.L., Schimmel, P. & Ewalt, K.L. Relationship of two human tRNA synthetases used in cell signaling. Trends Biochem. Sci. 29, 250–256 (2004).
Olink-Coux, M. & Hollenbeck, P.J. Localization and active transport of mRNA in axons of sympathetic neurons in culture. J. Neurosci. 16, 1346–1358 (1996).
Antonellis, A. et al. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am. J. Hum. Genet. 72, 1293–1299 (2003).
Corti, O. et al. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum. Mol. Genet. 12, 1427–1437 (2003).
Giuditta, A., Kaplan, B.B., van Minnen, J., Alvarez, J. & Koenig, E. Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci. 25, 400–404 (2002).
Barbarese, E. et al. Protein translation components are colocalized in granules in oligodendrocytes. J. Cell Sci. 108, 2781–2790 (1995).
Suter, U. & Scherer, S.S. Disease mechanisms in inherited neuropathies. Nat. Rev. Neurosci. 4, 714–726 (2003).
Zheng, J.Q. et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 21, 9291–9303 (2001).
Gaete, J., Kameid, G. & Alvarez, J. Regenerating axons of the rat require a local source of proteins. Neurosci. Lett. 251, 197–200 (1998).
Encinas, M. et al. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 75, 991–1003 (2000).
Van Damme, P., Callewaert, G., Eggermont, J., Robberecht, W. & Van den Bosch, L. Chloride influx aggravates Ca2+-dependent AMPA receptor-mediated motoneuron death. J. Neurosci. 23, 4942–4950 (2003).
Tanghe, A. et al. Aquaporin expression correlates with freeze tolerance in baker's yeast, and overexpression improves freeze tolerance in industrial strains. Appl. Environ. Microbiol. 68, 5981–5989 (2002).
De Corte, V. et al. Increased importin-β-dependent nuclear import of the actin modulating protein CapG promotes cell invasion. J. Cell Sci. 117, 5283–5292 (2004).
Kiga, D. et al. An engineered Escherichia coli tyrosyl-tRNA synthetase for site-specific incorporation of an unnatural amino acid into proteins in eukaryotic translation and its application in a wheat germ cell-free system. Proc. Natl. Acad. Sci. USA 99, 9715–9720 (2002).
Acknowledgements
We acknowledge the cooperation and participation of all patients and their relatives in this study. We thank the VIB Genetic Service Facility for genotyping and sequencing; C. Van Broeckhoven for support; V. De Corte and D. Adriaensen for discussions; J. Van Daele and D. De Rijck for assistance with confocal microscopy; C. Coun, C. Colombo and E. Vanderheyden for help with the yeast experiments; and B. Ishpekova and I. Litvinenko for clinical evaluation of the patients. This study was supported by the National Science Fund of the Bulgarian Ministry of Education and Science; the Fund for Scientific Research – Flanders (FWO); the Medical Foundation Queen Elisabeth; the Universities of Antwerp, Leuven and Ghent; and the Interuniversity Attraction Poles program of the Belgian Federal Science Office (POD). Additional support was provided by the Muscular Dystrophy Association (to A.J. and V.T.), the Neuropathy Association (to F.P.T.), the Association Belge contre les Maladies Neuromusculaires (to V.T.) and the Specific Support Action program of the European Union (to A.J. and I.K.). A.J. received visiting research fellowships from the POD and the North Atlantic Treaty Organisation/FWO. J.I. and K.M. are postdoctoral fellows of the FWO, and I.D. is a PhD fellow of the Institute for Science and Technology, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Heterodimerization between wild-type and mutant YARS isoforms. (PDF 108 kb)
Supplementary Fig. 2
Distribution of synaptophysin, mitochondria and α-tubulin in transfected N2a cells. (PDF 125 kb)
Supplementary Fig. 3
Western blot analysis of YARS. (PDF 211 kb)
Rights and permissions
About this article
Cite this article
Jordanova, A., Irobi, J., Thomas, F. et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet 38, 197–202 (2006). https://doi.org/10.1038/ng1727
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1727
This article is cited by
-
Clinical genetics of Charcot–Marie–Tooth disease
Journal of Human Genetics (2023)
-
Clinical characteristics and proteome modifications in two Charcot-Marie-Tooth families with the AARS1 Arg326Trp mutation
BMC Neurology (2022)
-
Cis- and trans-resveratrol have opposite effects on histone serine-ADP-ribosylation and tyrosine induced neurodegeneration
Nature Communications (2022)
-
Four pedigrees with aminoacyl-tRNA synthetase abnormalities
Neurological Sciences (2022)
-
The recurrent missense mutation p.(Arg367Trp) in YARS1 causes a distinct neurodevelopmental phenotype
Journal of Molecular Medicine (2021)