Abstract
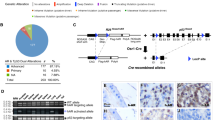
Cancer often results from the accumulation of multiple genetic alterations. Although most malignancies are sporadic, only a small number of genes have been shown to undergo frequent mutations in sporadic cancers. The long arm of chromosome 16 is frequently deleted in human cancers, but the target gene for this deletion has not been identified1,2,3,4. Here we report that ATBF1, which encodes a transcription factor that negatively regulates AFP and MYB5,6 but transactivates CDKN1A7, is a good candidate for the 16q22 tumor-suppressor gene. We narrowed the region of deletion at 16q22 to 861 kb containing ATBF1. ATBF1 mRNA was abundant in normal prostates but more scarce in approximately half of prostate cancers tested. In 24 of 66 (36%) cancers examined, we identified 22 unique somatic mutations, many of which impair ATBF1 function. Furthermore, ATBF1 inhibited cell proliferation. Hence, loss of ATBF1 is one mechanism that defines the absence of growth control in prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Knuutila, S. et al. DNA copy number losses in human neoplasms. Am. J. Pathol. 155, 683–694 (1999).
Dong, J.T. Chromosomal deletions and tumor suppressor genes in prostate cancer. Cancer Metastasis Rev. 20, 173–193 (2002).
Nelson, W.G., De Marzo, A.M. & Isaacs, W.B. Prostate cancer. N. Engl. J. Med. 349, 366–381 (2003).
Albertson, D.G., Collins, C., McCormick, F. & Gray, J.W. Chromosome aberrations in solid tumors. Nat. Genet. 34, 369–376 (2003).
Ninomiya, T. et al. Regulation of the alpha-fetoprotein gene by the isoforms of ATBF1 transcription factor in human hepatoma. Hepatology 35, 82–87 (2002).
Kaspar, P. et al. Myb-interacting protein, ATBF1, represses transcriptional activity of Myb oncoprotein. J. Biol. Chem. 274, 14422–14428 (1999).
Kataoka, H. et al. ING1 represses transcription by direct DNA binding and through effects on p53. Cancer Res. 63, 5785–5792 (2003).
Latil, A., Cussenot, O., Fournier, G., Driouch, K. & Lidereau, R. Loss of heterozygosity at chromosome 16q in prostate adenocarcinoma: identification of three independent regions. Cancer Res. 57, 1058–1062 (1997).
Morinaga, T., Yasuda, H., Hashimoto, T., Higashio, K. & Tamaoki, T. A human alpha-fetoprotein enhancer-binding protein, ATBF1, contains four homeodomains and seventeen zinc fingers. Mol. Cell. Biol. 11, 6041–6049 (1991).
Kataoka, H. et al. Alpha-fetoprotein producing gastric cancer lacks transcription factor ATBF1. Oncogene 20, 869–873 (2001).
Miura, Y. et al. Cloning and characterization of an ATBF1 isoform that expresses in a neuronal differentiation-dependent manner. J. Biol. Chem. 270, 26840–26848 (1995).
Kawaguchi, M. et al. DNA/RNA-dependent ATPase activity is associated with ATBF1, a multiple homeodomain-zinc finger protein. Biochim. Biophys. Acta 1550, 164–174 (2001).
Ionov, Y., Peinado, M.A., Malkhosyan, S., Shibata, D. & Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363, 558–561 (1993).
Coolidge, C.J., Seely, R.J. & Patton, J.G. Functional analysis of the polypyrimidine tract in pre-mRNA splicing. Nucleic Acids Res. 25, 888–896 (1997).
Remacle, J.E., Albrecht, G., Brys, R., Braus, G.H. & Huylebroeck, D. Three classes of mammalian transcription activation domain stimulate transcription in Schizosaccharomyces pombe. EMBO J. 16, 5722–5729 (1997).
Takada, N., Ozaki, T., Ichimiya, S., Todo, S. & Nakagawara, A. Identification of a transactivation activity in the COOH-terminal region of p73 which is impaired in the naturally occurring mutants found in human neuroblastomas. Cancer Res. 59, 2810–2814 (1999).
Courey, A.J., Holtzman, D.A., Jackson, S.P. & Tjian, R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell 59, 827–836 (1989).
Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P.P. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 (1998).
Strup, S.E. et al. Chromosome 16 allelic loss analysis of a large set of microdissected prostate carcinomas. J. Urol. 162, 590–594 (1999).
Matsuyama, H. et al. Clinical significance of chromosome 8p, 10q, and 16q deletions in prostate cancer. Prostate 54, 103–111 (2003).
Ido, A., Miura, Y. & Tamaoki, T. Activation of ATBF1, a multiple-homeodomain zinc-finger gene, during neuronal differentiation of murine embryonal carcinoma cells. Dev. Biol. 163, 184–187 (1994).
Ishii, Y. et al. ATBF1-A protein, but not ATBF1-B, is preferentially expressed in developing rat brain. J. Comp. Neurol. 465, 57–71 (2003).
Chen, C., Bhalala, H.V., Vessella, R.L. & Dong, J.T. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate 55, 81–88 (2003).
Dong, J.T., Chen, C., Stultz, B.G., Isaacs, J.T. & Frierson, H.F., Jr. Deletion at 13q21 is associated with aggressive prostate cancers. Cancer Res. 60, 3880–3883 (2000).
Chen, C. et al. An 800 kb region of deletion at 13q14 in human prostate and other carcinomas. Genomics 77, 135–144 (2001).
Goldberg, E.K. et al. Localization of multiple melanoma tumor-suppressor genes on chromosome 11 by use of homozygosity mapping-of-deletions analysis. Am. J. Hum. Genet. 67, 417–431 (2000).
Chen, C. et al. Deletion, mutation, and loss of expression of KLF6 in human prostate cancer. Am. J. Pathol. 162, 1349–1354 (2003).
Kukita, Y., Tahira, T., Sommer, S.S. & Hayashi, K. SSCP analysis of long DNA fragments in low pH gel. Hum. Mutat. 10, 400–407 (1997).
Sun, S.Y. et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 57, 4931–4939 (1997).
Acknowledgements
We thank L.W.K. Chung for his encouragement. This work was supported by grants from the National Cancer Institute, Department of Defense Prostate Cancer Research Program, and the Georgia Cancer Coalition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Examples of sequencing results showing the affected nucleotide in a case. (PDF 188 kb)
Supplementary Table 1
Short mononucleotide tracts and their alterations in tumor and normal samples. (PDF 18 kb)
Supplementary Table 2
Summary of nucleotide alterations considered as polymorphisms. (PDF 22 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Frierson, H., Chen, C. et al. Frequent somatic mutations of the transcription factor ATBF1 in human prostate cancer. Nat Genet 37, 407–412 (2005). https://doi.org/10.1038/ng1528
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1528
This article is cited by
-
Genomic approaches to identify and investigate genes associated with atrial fibrillation and heart failure susceptibility
Human Genomics (2023)
-
Genomic and epigenomic integrative subtypes of renal cell carcinoma in a Japanese cohort
Nature Communications (2023)
-
Evolutionary signatures of human cancers revealed via genomic analysis of over 35,000 patients
Nature Communications (2023)
-
Androgen receptor binding sites enabling genetic prediction of mortality due to prostate cancer in cancer-free subjects
Nature Communications (2023)
-
Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants
Nature Genetics (2023)