Abstract
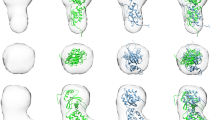
The X–lined gene for Norrie disease, which is characterized by blindness, deafness and mental retardation has been cloned recently. This gene has been thought to code for a putative extracellular factor; its predicted amino acid sequence is homologous to the C–terminal domain of diverse extracellular proteins. Sequence pattern searches and three–dimensional modelling now suggest that the Norrie disease protein (NDP) has a tertiary structure similar to that of transforming growth factor β (TGFβ). Our model identifies NDP as a member of an emerging family of growth factors containing a cystine knot motif, with direct implications for the physiological role of NDP. The model also sheds light on sequence related domains such as the C–terminal domain of mucins and of von Willebrand factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sporn, M.B. & Roberts, A.B. (eds) In Peptide growth factors and their receptors (Springer, Berlin 1990).
McDonald, N.Q. & Hendrickson, W.A. A structural superfamily of growth factors containing a cystine knot motif. Cell 73, 421–424 (1993).
Angeletti, R.H. & Bradshaw, R.A. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc. natn. Acad. Sci. U.S.A. 68, 2417–2420 (1971).
McDonald, N.Q. et al. New protein fold revealed by a 2,3-Å resolution crystal structure of nerve growth factor. Nature 364, 411–414 (1991).
Schlunegger, M.P. & Grutter, M.G. An unusual feature revealed by the crystal structure at 2,2Å resolution of human transforming growth factor-β2. Nature 358, 430–434 (1992).
Daopin, S., Piez, K.A., Ogawa, Y. & Davies, D.R. Crystal structure of transforming growth factor β2: An unusual fold for the superfamily. Science 257, 369–373 (1992).
Oefner, C., D'Arcy, A., Winkler, F.K., Eggiman, B. & Hosang, M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 11, 3921–3926 (1992).
Swindells, M.B., Daopin, S., Cohen, G.H. & Davies, D. Structural similarity between transforming growth factor. ß2 and nerve growth factor Science 258, 1160–1162 (1992).
Lin, L-F.H. et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 (1993).
Meindl, A. et al. Norrie disease is caused by mutations in an extracellular protein resembling C-terminal globular domain of mucins. Nature Genet 2, 139–143 (1992).
Berger, W. et al. Isolation of a candidate gene for Norrie disease by positional cloning. Nature Genet 1, 199–203 (1992).
Chen, Z-Y. et al. Isolation and characterisation of a candidate gene for Norrie disease. Nature Genet 1, 204–208 (1992).
Berger, W. et al. Mutations in the candidate gene for Norrie disease. Hum. molec. Genet. 1, 461–465, (1992).
Warburg, M. Norrie's disease: A congenital progressive oculo-acoustico-cerebral degeneration. Acta Ophthalmol. 89, 1–147 (1966).
Apple, D.J., Fishman, G.A. & Goldberg, M.F. Ocular histopathology of Norrie's disease. Am. J. Ophthalmol. 78, 196–203 (1974).
Parsons, M.A., Curtis, D., Blank, C.E., Hughes, H.N. & McCatney, A.C.E. The ocular pathology of Norrie disease in a fetus of 11 weeks' gestational age. Graefe's Arch. clin. exp. Ophthalmol. 230, 248–251 (1992).
Rothberg, J.M. & Artavanis-Tsakonas, S. Modularity of the slit protein: characterisation of a conserved carboxy-terminal sequence in secreted proteins and a motif implicated in extracellular protein interactions. J. molec. Biol. 227, 367–370 (1992).
Mancuso, D.J. et al. Structure and gene for human von Willebrand factor. J. biol. Chem. 264, 19514–19527 (1989).
Rothberg, J.M., Jacobs, J.R., Goodman, C.S. & Atavanis-Tsakonas, S. Slit: an extracellular protein necessary for development of midline glia and commisural axon pathways contains both EGF and LRR domains. Genes Dev. 4, 2169–2187 (1990).
Bradham, D.M., Igarashi, A., Potter, R.L. & Grotendorst, G.R. Connecting tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J. cell Biol. 114, 1285–1294 (1986).
Rohde, K. & Bork, P. A fast, sensitive pattern matching approach for protein sequences. Comp. Appl. Biosci. 6, 183–189 (1993).
Rost, B. & Sander, C. Prediction of protein structure at better than 70% accuracy. J. molec. Biol. 232, 584–599 (1993).
Voorberg, J. et al. Assembly and routing of von Willebrand factor variants: the requirements for disulphide-linked dimerization reside within the carboxy-terminal 151 amino acids. J. cell Biol. 113, 195–205 (1991).
Tamaoki, H. et al. Solution conformation of endothelin determined by means of 1H-NMR spectroscopy and distance geometry calculations. Protein Eng. 4, 509–518 (1991).
Qian, S.W. et al. Identification of a structural domain that distinguishes the actions of the type 1 and 2 isoforms of transforming growth factos ß on endothelial cells. Proc. natn. Acad. Sci. U.S.A. 89, 6290–6294 (1992).
Steel, C.M. Peptide regulatory factors and malignancy in Peptide regulatory factors p. 121 (Edward Arnold, London, 1989).
Arend, W.P. & Dayer, J.M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 33, 305 (1990).
Kousseff, B.G. Sipple syndrome with lichen amyloidosis as a paracrinopathy. Am. J. med. Genet. 42, 751–753 (1992).
Luetteke, N.C. et al. TGFa deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73, 263–278 (1993).
Skevas, A., Kastanioudakis, I., Daniilidis, B. & Exarchakos, G. Norrie Warburg Syndrom. Laryngo-Rhino-Otol. 71, 534–536 (1992).
Mudge, A.W. Motor neurons find their factors. Nature 363, 213 (1993).
LaVail, M. et al. Multiple growth factors, cytokines and neurotrophins rescue photoreceptors form the damaging effects of constant light. Proc. natn. Acad. Sci. U.S.A. 89, 11249–11253 (1992).
Pearson, W.R. & Lipman, D.J. Improved tools for biological sequence comparison. Proc. natn. Acad. Sci. U.S.A. 85, 2444–2448 (1988).
Argos, P. A sensitive procedure to compare amino acid sequences. J. molec. Biol. 193, 385–391 (1988).
Rost, B., Schneider, R. & Sander, C. Progress in protein structure prediction? Trends Biochem. Sci. 18, 120–123 (1993).
Bemstein, S.C. et al. The protein databank: computer based archival file for macromolecular studies. J. molec. Biol. 112, 535–542 (1978).
Vriend, G. WHAT IF: a molecular modelling and drug design program J. molec. Graphics 8, 52–56 (1990).
Weiner, S.J. et al. A new force field for molecular simulation of nucleic acids and proteins. J. Am. chem. Soc. 106, 765–784 (1984).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed a schematic plots of protein structures. J. appl. Crystallogr. 24, 946–950 (1991).
Gum, J.R. et al. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J. biol. Chem. 267, 21375–21383 (1992).
Xu, G. et al. cDNA for the carboxyl-terminal region of a rat intestinal mucin-like peptide. J. biol. Chem. 267, 5401–5407 (1992).
Bork, P. Mobile modules and motifs. Curr. Op. struc. Biol. 2, 413–421 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meitinger, T., Meindl, A., Bork, P. et al. Molecular modelling of the Norrie disease protein predicts a cystine knot growth factor tertiary structure. Nat Genet 5, 376–380 (1993). https://doi.org/10.1038/ng1293-376
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1293-376
This article is cited by
-
Whole exome sequencing revealed 14 variants in NDP, FZD4, LRP5, and TSPAN12 genes for 20 families with familial exudative vitreoretinopathy
BMC Medical Genomics (2022)
-
Novel Norrie disease gene mutations in Chinese patients with familial exudative vitreoretinopathy
BMC Ophthalmology (2021)
-
Modeling the human intestinal Mucin (MUC2) C-terminal cystine knot dimer
Journal of Molecular Modeling (2011)
-
Wnt/Frizzled signaling in angiogenesis
Angiogenesis (2008)
-
Phenotypic heterogeneity associated with a novel mutation (Gly112Glu) in the Norrie disease protein
Eye (2006)