Abstract
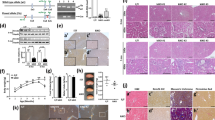
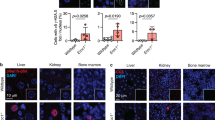
p53R2, which is regulated by tumor suppressor p53, is a small subunit of ribonucleotide reductase1. To determine whether it is involved in DNA repair by supplying deoxyribonucleotides (dNTPs) for resting cells in vivo2,3,4,5, we generated a strain of mice lacking Rrm2b (encoding p53R2). These mice developed normally until they were weaned but from then on had growth retardation and early mortality. Pathological examination indicated that multiple organs had failed, and all Rrm2b-null mice died from severe renal failure by the age of 14 weeks. TUNEL staining showed a greater number of apoptotic cells in kidneys of 8-week-old Rrm2b−/− mice relative to wild-type mice. p53 was activated in kidney tissues of Rrm2b−/− mice, leading to transcriptional induction of p53 target genes. Rrm2b−/− mouse embryonic fibroblasts (MEFs) became immortal much earlier than Rrm2b+/+ MEFs. dNTP pools were severely attenuated in Rrm2b−/− MEFs under oxidative stress. Rrm2b deficiency caused higher rates of spontaneous mutation in the kidneys of Rrm2b−/− mice. Our results suggest that p53R2 has a pivotal role in maintaining dNTP levels for repair of DNA in resting cells. Impairment of this pathway may enhance spontaneous mutation frequency and activate p53-dependent apoptotic pathway(s) in vivo, causing severe renal failure, growth retardation and early mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tanaka, H. et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404, 42–49 (2000).
Lozano, G. & Elledge, S.J. p53 sends nucleotides to repair DNA. Nature 404, 24–25 (2000).
Jordan, A. & Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 67, 71–98 (1998).
Yamaguchi, T. et al. p53R2-dependent pathway for DNA synthesis in a p53-regulated cell cycle checkpoint. Cancer Res. 61, 8256–8262 (2001).
Zhou, B.B. & Elledge, S.J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Oda, K. et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102, 849–862 (2000).
Matsuda, K., Yoshida, K., Nakamura, K., Nakamura, Y. & Arakawa, H. p53AIP1 regulates the mitochondrial apoptotic pathway. Cancer Res. 62, 2883–2889 (2002).
Harper, J.W., Adami, G.R., Wei, N., Keyomarsi, K. & Elledge, S.J. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
el-Deiry, W.S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Miyashita, T. & Reed, J.C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-dependent apoptosis. Science 288, 1053–1058 (2000).
Okamura, S. et al. p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis. Mol. Cell 8, 85–94 (2001).
Guittet, O. et al. Mammalian p53R2 protein forms an active ribonucleotide reductase in vitro with the R1 protein, which is expressed both in resting cells in response to DNA damage and in proliferating cells. J. Biol. Chem. 276, 40647–40651 (2001).
Nohmi, T., Suzuki, T. & Masumura, K. Recent advances in the protocols of transgenic mouse mutation assays. Mutat. Res. 455, 191–215 (2000).
Chabes, A. et al. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112, 391–401 (2003).
Elledge, S.J. & Davis, R.W. Identification and isolation of the gene encoding the small subunit of ribonucleotide reductase from Saccharomyces cerevisiae: a DNA damage inducible gene required for mitotic viability. Mol. Cell. Biol. 7, 2783–2793 (1987).
Elledge, S.J. & Davis, R.W. DNA damage induction of ribonucleotide reductase. Mol. Cell. Biol. 9, 4932–4940 (1989).
Elledge, S.J. & Davis, R.W. Identification of the DNA damage-responsive element of RNR2 and evidence that four distinct cellular factors bind it. Mol. Cell. Biol. 9, 5373–5386 (1989).
Vogelstein, B., Lane, D. & Levine, A.J. Surfing the p53 network. Nature 408, 307–310 (2000).
Todaro, G.J. & Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell. Biol. 17, 299–313 (1963).
Sherman, P.A. & Fyfe, J.A. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal. Biochem. 180, 222–226 (1989).
Acknowledgements
We thank Y. Fujisawa, Y. Anazawa, Y. Kikuta, N. Nishino, T. Wadatsu, T. Sato and N. Miyazawa for technical assistance. This work was supported in part by Research for the Future Program Grant from the Japan Society for the Promotion of Science (to Y.N.) and in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology (to H.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Kimura, T., Takeda, S., Sagiya, Y. et al. Impaired function of p53R2 in Rrm2b-null mice causes severe renal failure through attenuation of dNTP pools. Nat Genet 34, 440–445 (2003). https://doi.org/10.1038/ng1212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1212
This article is cited by
-
Comprehensive assessment of the genetic characteristics of small for gestational age newborns in NICU: from diagnosis of genetic disorders to prediction of prognosis
Genome Medicine (2023)
-
Prolonged Exposure to High Glucose Induces Premature Senescence Through Oxidative Stress and Autophagy in Retinal Pigment Epithelial Cells
Archivum Immunologiae et Therapiae Experimentalis (2023)
-
Ribonucleotide reductase M2B in the myofibers modulates stem cell fate in skeletal muscle
npj Regenerative Medicine (2022)
-
Rrm2b deletion causes mitochondrial metabolic defects in renal tubules
Scientific Reports (2019)
-
p53R2 as a novel prognostic biomarker in nasopharyngeal carcinoma
BMC Cancer (2017)