Abstract
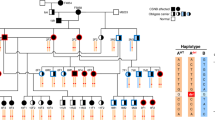
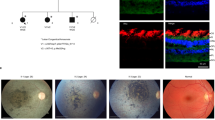
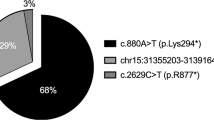
During development, visual photoreceptors, bipolar cells and other neurons establish connections within the retina enabling the eye to process visual images over approximately 7 log units of illumination1. Within the retina, cells that respond to light increment and light decrement are separated into ON- and OFF-pathways. Hereditary diseases are known to disturb these retinal pathways, causing either progressive degeneration or stationary deficits2. Congenital stationary night blindness (CSNB) is a group of stable retinal disorders that are characterized by abnormal night vision. Genetic subtypes of CSNB have been defined and different disease actions have been postulated3,4,5. The molecular bases have been elucidated in several subtypes, providing a better understanding of the disease mechanisms and developmental retinal neurobiology2. Here we have studied 22 families with 'complete' X-linked CSNB (CSNB1; MIM 310500; ref. 4) in which affected males have night blindness, some photopic vision loss and a defect of the ON-pathway. We have found 14 different mutations, including 1 founder mutation in 7 families from the United States, in a novel candidate gene, NYX. NYX, which encodes a glycosylphosphatidyl (GPI)-anchored protein called nyctalopin, is a new and unique member of the small leucine-rich proteoglycan (SLRP) family6. The role of other SLRP proteins suggests that mutant nyctalopin disrupts developing retinal interconnections involving the ON-bipolar cells, leading to the visual losses seen in patients with complete CSNB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rodieck, R.W. The First Steps in Seeing (Sinauer, Sunderland, Massachusetts, 1998).
Rattner, A., Sun, H. & Nathans, J. Molecular genetics of human retinal disease. Annu. Rev. Genet. 33, 85–131 (1999).
Carr, R.E. Congenital stationary night blindness. Trans. Am. Ophthalmol. Soc. 72, 448–487 (1974).
Miyake, Y., Yagasaki, K., Horiguchi, M., Kawase, Y. & Kanda, T. Congenital stationary night blindness with negative eletroretinogram. Arch. Ophthalmol. 104, 1013–1020 (1986).
Sharp, D.M., Arden, G.B., Kemp, C.M., Hogg, C.R. & Bird, A.C. Mechanisms and sites of loss of scotopic sensitivity: a clinical analysis of congenital stationary night blindness. Clin. Vis. Sci. 5, 217–230 (1990).
Hocking, A.M., Shinomura, T. & McQuillan, D.J. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 17, 1–19 (1998).
Schubert, G. & Bornschein, H. Beitrag zur Analyse des menschlichen Elektroretinogramms. Ophthalmologica 123, 396–413 (1952).
Miyake, Y., Yagasaki, K., Horiguchi, M. & Kawase, Y. On- and Off-responses in photopic electroretinogram in complete and incomplete types of congenital stationary night blindness. Jpn. J. Ophthalmol. 31, 81–87 (1987).
Young, R.S.L. Low-frequency component of the photopic ERG in patients with the X-linked congenital stationary night blindness. Clin. Vision Sci. 6, 309–315 (1991).
Young, R.S.L., Price, J. & Harrison, J. Psychophysical study of rod adaptation in patients with congenital stationary night blindness. Clin. Vis. Sci. 1, 137–143 (1986).
Jacobson, S.G. et al. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology 93, 1604–1611 (1986).
Boycott, K.M. et al. Evidence for genetic heterogeneity in X-linked congenital stationary night blindness. Am. J. Hum. Genet. 62, 865–875 (1998).
Bergen, A.A.B., ten Brink, J.B., Riemslag, F., Schuurman, E.J.M. & Tijmes, N. Localization of a novel X-linked congenital stationary night blindness locus: close linkage to the RP3 type retinitis pigmentosa gene region. Hum. Mol. Genet. 4, 931–935 (1995).
Sparkes, R.L., Summer, C., Boycott, K.M., Zahorchak, R.J. & Bech-Hansen, N.T. Development of a high resolution 1.4-Megabase BAC/PAC contig and physical map of the candidate region for complete X-linked congenital stationary night blindness in Xp11.4. Genomics 68, 77–100 (2000).
Kobe, B. & Deisenhofer, J. The leucine-rich repeat: a versatile binding motif. Trends Biol. Sci. 19, 415–421 (1994).
Plaas, A.H.K., Meame, P.J., Nivens, C.M. & Reiss, L. Identification of the keratan sulfate attachment sites on bovine fibromodulin. J. Biol. Chem. 265, 20634–20640 (1990).
Nielsen, H., Engelbrecht, J., Brunak, S. & von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Prot. Eng. 10, 1–6 (1997).
Englund, P.T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu. Rev. Biochem. 62, 121–138 (1993).
Krantz, D.E. & Zipursky, S.L. Drosophila chaoptin, a nember of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. EMBO J. 9, 1969–1977 (1990).
Shishido, E., Takeichi, M. & Nose, A. Drosophila synapse formation: regulation by transmembrane protein with leu-rich repeats, CAPRICIOUS. Science 280, 2118–2121 (1998).
Vainio, S. & Muller, U. Inductive tissue interactions, cell signaling, and the control of kidney organogenesis. Cell 90, 975–978 (1997).
Bech-Hansen, N.T. et al. Loss-of-function mutations in a calcium-channel α1-subunit in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genet. 19, 264–267 (1998).
Strom, T. et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genet. 19, 260–263 (1998).
Candille, S.I., Pardue, M.T., McCall, M.A., Peachey, N.S. & Gregg, R.A. Localization of the mouse nob (no b-wave) gene to the centromeric region of the X chromosome. Invest. Ophthalmol. Vis. Sci. 40, 2748–2751 (1999).
Pusch, C.M. et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nature Genet. 26, 324–327 (2000).
Pellegate, N.S. et al. Mutations in KERA, encoding keratocan, cause cornea plana. Nature Genet. 25, 91–95 (2000).
Musarella, M.A. et al. Assignment of the gene for complete X-linked congenital stationary night blindness (CSNB1) to human chromosome Xp11.3. Genomics 5, 727–737 (1989).
Bergen, A.A.A. et al. Conclusive evidence for a distinct congenital stationary night blindness locus in Xp21.1. J. Med. Genet. 33, 869–872 (1996).
Gal, A. et al. Gene of X-chromosomal congenital stationary night blindness is closely linked to DXS7 on Xp. Hum. Genet. 81, 315–318 (1989).
Bech-Hansen, N.T., Boycott, K.M., Gratton, K.J., Ross, D. & Pearce, W.G. Localization of a gene for incomplete X-linked congenital stationary night blindness in Xp11.23 to the interval betweeen DXS6849 and DXS8023. Hum. Genet. 103, 124–130 (1998).
Rowen, L. & Koop, B.F. in Automated DNA Sequencing and Analysis (eds Adams, M.D., Fields, C. & Venter, J.C.) 167–174 (Academic, London and San Diego, 1994).
Prinsen, C.F.M., Szerencsei, R.T. & Schnetkamp, P.P.M. Molecular cloning and functional expression of the potassium-dependent sodium-calcium exchanger from human and chicken retinal cone photoreceptors. J. Neurosci. 20, 1424–1434 (2000).
Antonorakis, S.E. et al. Recommendations for a nomenclature system for human gene mutations. Hum. Genet. 11, 1–3 (1998).
Acknowledgements
We thank the patients and their families for participation; K. Boycott, J. Friedman, D. Rancourt, S. Robbins, P. Schnetkamp, W. Stell, M. Walter and R. Winkfein for discussions; and J. Whitehead, D. Martindale and L. Yong for technical assistance. This research was supported in part by operating grants to N.T.B.-H. from the RP Research Foundation (Foundation Fighting Blindness), the Medical Research Council of Canada, and the I.D. Bebensee Foundation, and from the Foundation Fighting Blindness to D.G.B., S.G.J. and R.G.W. In addition, R.G.W. was supported by an unrestricted grant from Research to Prevent Blindness, S.G.J. by NIH grant EY-05627 and D.G.B. by NIH grant EY05235. N.T.B.-H. was also supported by the Roy Allen Endowment, The Alberta Children's Hospital Foundation and the Department of Ophthalmology, University of Alberta (W.G. Pearce and I.M. MacDonald).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bech-Hansen, N., Naylor, M., Maybaum, T. et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet 26, 319–323 (2000). https://doi.org/10.1038/81619
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81619
This article is cited by
-
Sex differences in the intergenerational link between maternal and neonatal whole blood DNA methylation: a genome-wide analysis in 2 birth cohorts
Clinical Epigenetics (2023)
-
Gastroprotective and ulcer healing potentials of Nigerian Bee Propolis flavonoid extract on acetic acid-induced gastric ulcers in albino rats (Wistar Strains)
Advances in Traditional Medicine (2023)
-
Comparing the RETeval® portable ERG device with more traditional tabletop ERG systems in normal subjects and selected retinopathies
Documenta Ophthalmologica (2023)
-
The inner junction protein CFAP20 functions in motile and non-motile cilia and is critical for vision
Nature Communications (2022)
-
Genome-wide analysis of retinal transcriptome reveals common genetic network underlying perception of contrast and optical defocus detection
BMC Medical Genomics (2021)