Abstract
The repair-deficient form of trichothiodystrophy (TTD) most often results from mutations in the genes XPB or XPD, encoding helicases of the transcription/repair factor TFIIH. The genetic defect in a third group, TTD-A, is unknown, but is also caused by dysfunctioning TFIIH. None of the TFIIH subunits carry a mutation and TFIIH from TTD-A cells is active in both transcription and repair. Instead, immunoblot and immunofluorescence analyses reveal a strong reduction in the TFIIH concentration. Thus, the phenotype of TTD-A appears to result from sublimiting amounts of TFIIH, probably due to a mutation in a gene determining the complex stability. The reduction of TFIIH mainly affects its repair function and hardly influences transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Friedberg, E.C., Walker, G.C. & Siede, W. DNA Repair and Mutagenesis (ASM, Washington, DC, 1995).
De Laat, W.L., Jasper, N.G. & Hoeijmakers, J.H. Molecular mechanism of nucleotide excision repair . Genes Dev. 13, 768–785 (1999).
Araujo, S.J. et al. Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev. 14, 349– 359 (2000).
Hanawalt, P.C. The bases for Cockayne syndrome. Nature 405, 415–416 (2000).
Schaeffer, L. et al. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science 260, 58– 63 (1993).
Sung, P., Guzder, S.N., Prakash, L. & Prakash, S. Reconstitution of TFIIH and requirement of its DNA helicase subunits, Rad3 and Rad25, in the incision step of nucleotide excision repair. J. Biol. Chem. 271, 10821–10826 (1996).
Feaver, W.J. et al. Dual roles of a multiprotein complex from S. cerevisiae in transcription and DNA repair. Cell 75, 1379 –1387 (1993).
Drapkin, R. et al. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature 368, 769– 772 (1994).
Bootsma, D., Kraemer, K.H., Cleaver, J.E. & Hoeijmakers, J.H.J. Nucleotide Excision Repair Syndromes: Xeroderma Pigmentosum, Cockayne Syndrome, And Trichothiodystrophy 245–274 (Mc Graw-Hill, New York, 1998).
Broughton, B.C. et al. Molecular and cellular analysis of the DNA repair defect in a patient with xeroderma pigmentosum complementation group D with the clinical features of xeroderma pigmentosum and Cockayne syndrome. Am. J. Hum. Genet. 56, 167–174 (1995).
Taylor, E. et al. Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc. Natl Acad. Sci. USA 94, 8658– 8663 (1997).
de Boer, J. & Hoeijmakers, J.H. Nucleotide excision repair and human syndromes. Carcinogenesis 21, 453–460 (2000).
Weeda, G. et al. A mutation in the XPB/ERCC3 DNA repair transcription gene, associated with trichothiodystrophy. Am. J. Hum. Genet. 60, 320–329 (1997).
Bootsma, D. & Hoeijmakers, J.H.J. DNA repair. Engagement with transcription. Nature 363, 114–115 (1993).
Lehmann, A.R. Dual functions of DNA repair genes: molecular, cellular, and clinical implications . Bioessays 20, 146–155 (1998).
de Boer, J. et al. Mouse model for the DNA repair/basal transcription disorder trichothiodystrophy reveals cancer predisposition. Cancer Res. 59, 3489–3494 ( 1999).
Hwang, B.J., Liao, J.C. & Chu, G. Isolation of a cDNA encoding a UV-damaged DNA binding factor defective in xeroderma pigmentosum group E cells. Mutat. Res. 362, 105–117 ( 1996).
Coin, F. et al. Mutations in the XPD helicase result in XP and TTD phenotypes, preventing the interaction of XPD with the p44 subunit of TFIIH. Nature Genet. 20, 184–188 (1998).
Coin, F., Bergmann, E., Tremeau-Bravard, A. & Egly, J.M. Mutations in XPB and XPD helicases found in xeroderma pigmentosum patients impair the transcription function of TFIIH. EMBO J. 18, 1357–1366 (1999).
Bradsher, J., Coin, F. & Egly, J.M. Distinct roles for the helicases of TFIIH in transcript initiation and promoter escape. J. Biol. Chem. 275, 2532–2538 (2000).
Moreland, R.J. et al. A role for the TFIIH XPB DNA helicase in promoter escape by RNA polymerase II. J. Biol. Chem. 274, 22127 –22130 (1999).
Stefanini, M. et al. A new nucleotide-excision repair gene associated with the disorder trichothiodystrophy. Am. J. Hum. Genet. 53 , 817–821 (1993).
Vermeulen, W. et al. Three unusual repair deficiencies associated with transcription factor BTF2 (TFIIH). Evidence for the existence of a transcription syndrome . Cold Spring Harb. Symp. Quant. Biol. 59, 317–329 (1994).
Marinoni, J.C. et al. Cloning and characterization of p52, the fifth subunit of the core of the transcription/DNA repair factor TFIIH. EMBO J. 16, 1093–1102 ( 1997).
Tirode, F., Busso, D., Coin, F. & Egly, J. Reconstitution of the transcription factor TFIIH: assignment of the functions for the three enzymatic subunits, XPB, XPD and cdk7. Mol. Cell 3, 87–95 (1999).
Vermeulen, W. et al. Clinical heterogeneity within xeroderma pigmentosum associated with mutations in the DNA repair and transcription gene ERCC3. Am. J. Hum. Genet. 54, 191–200 (1994).
Winkler, G.S. et al. TFIIH with inactive XPD helicase functions in transcription initiation but is defective in DNA repair. J. Biol. Chem. 275, 4258–4266 (2000).
Roy, R. et al. The DNA-dependent ATPase activity associated with the class II transcription factor BTF2/TFIIH. J. Biol. Chem. 269 , 9826–9832 (1994).
Evans, E., Fellows, J., Coffer, A. & Wood, R.D. Open complex formation around lesion during nucleotide excision repair provides a structure for cleavage by human XPG protein. EMBO J. 16, 625– 638 (1997).
Sijbers, A.M. et al. Mutational analysis of the human nucleotide excision repair gene ERCC1. Nucleic Acids Res. 24, 3370– 3380 (1996).
Singleton, B.K., Torres-Arzayus, M.I., Rottinghaus, S.T., Taccioli, G.E. & Jeggo, P.A. The C terminus of Ku80 activates the DNA-dependent protein kinase catalytic subunit. Mol. Cell. Biol. 19, 3267– 3277 (1999).
Waisfisz, Q. et al. A physical complex of the Fanconi anemia proteins FANCG/XRCC9 and FANCA. Proc. Natl Acad. Sci. USA 96, 10320–10325 (1999).
Winkler, G.S. et al. Affinity purification of human DNA repair/transcription factor TFIIH using epitope-tagged xeroderma pigmentosum B protein. J. Biol. Chem. 273, 1092–1098 (1998).
Weeda, G. et al. The XPB subunit of repair/transcription factor TFIIH directly interacts with SUG1, a subunit of the 26S proteasome and putative transcription factor. Nucleic Acids Res. 25, 2274– 2283 (1997).
van Oosterwijk M.F. et al. Lack of transcription-coupled repair of acetylaminofluorene DNA adducts in human fibroblasts contrasts their efficient inhibition of transcription . J. Biol. Chem. 273, 13599– 13604 (1998).
Svejstrup, J.Q. et al. Different forms of TFIIH for transcription and DNA repair: holo-TFIIH and a nucleotide excision repairosome. Cell 80, 21–28 (1995).
Satoh, M.S. & Hanawalt, P.C. Competent transcription initiation by RNA polymerase II in cell free extracts from xeroderma pigmentosum groups B and D in an optimized RNA transcription assay. Biochim. Biophys. Acta 1354, 241–251 (1997).
Satoh, M.S. & Hanawalt, P.C. TFIIH-mediated nucleotide excision repair and initiation of mRNA transcription in an optimized cell-free DNA repair and RNA transcription assay. Nucleic Acids Res. 24, 3576–3582 (1996).
Houtsmuller A.B., . et al. Action of DNA repair endonuclease ERCC1/XPF in living cells . Science 284, 958–961 (1999).
Gerard, M. et al. Purification and interaction properties of the human RNA polymerase B(II) general transcription factor BTF2. J. Biol. Chem. 266, 20940–20945 (1991).
Sambrook, J., Fritsch, E. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989).
Acknowledgements
We thank D. Bootsma for support; F. Coin, N.G.J. Jaspers, A. Lehman, M. Stefanini, G.S. Winkler and G. Weeda for discussions; A. Fery and J.L. Weickert for technical assistance; A. Raams for cell culture; and S. Vicaire for DNA sequencing. E.B. was supported by a Ligue Nationale Contre le Cancer fellowship, J.A. by la Fondation pour la Recherche Médicale. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Hôpital Universitaire de Strasbourg, by HFSP and EEC grants to both J.M.E. and J.H.J.H., the Association pour la Recherche sur le Cancer, by the Dutch Cancer Society, a NIH programme to J.H.J.H., the Research Institute for Diseases in the Elderly, funded by the Ministry of Education & Science and the Ministry of Health, Welfare and Sports, through the Netherlands Organization for Scientific Research (NWO) and the Louis Jeantet Foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vermeulen, W., Bergmann, E., Auriol, J. et al. Sublimiting concentration of TFIIH transcription/DNA repair factor causes TTD-A trichothiodystrophy disorder. Nat Genet 26, 307–313 (2000). https://doi.org/10.1038/81603
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81603
This article is cited by
-
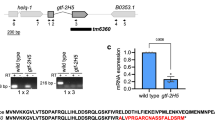
C. elegans TFIIH subunit GTF-2H5/TTDA is a non-essential transcription factor indispensable for DNA repair
Communications Biology (2021)
-
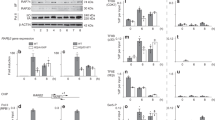
Cell-type specific concentration regulation of the basal transcription factor TFIIH in XPBy/y mice model
Cancer Cell International (2019)
-
TFIIE orchestrates the recruitment of the TFIIH kinase module at promoter before release during transcription
Nature Communications (2019)
-
A homozygous G insertion in MPLKIP leads to TTDN1 with the hypergonadotropic hypogonadism symptom
BMC Medical Genetics (2018)
-
DNA damage sensitivity of SWI/SNF-deficient cells depends on TFIIH subunit p62/GTF2H1
Nature Communications (2018)