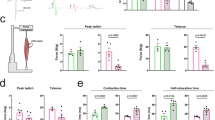
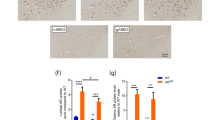
Abstract
Expansion of the long (CAG; glutamine)n repeat in the first exon of the X–linked human androgen receptor gene (hAR) causes spinal and bulbar muscular atrophy, frequently in association with mild androgen insensitivity. The relevant normal motor neurons are preferentially stimulated by androgen, however no motor neuron disorder occurs with any other known AR mutation, including those that cause complete androgen insensitivity. We have found that a polyglutamine (Gln) expanded AR transactivates an androgen–responsive reporter gene subnormally. Other groups have reported that a poly Gln–deleted AR transactivates normally. A parsimonious interpretation of all these facts is that poly Gln expansion causes the AR to lose a function that is necessary for full androgen sensitivity and to gain a function that is selectively motor neuronotoxic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kennedy, W.R., Alter, M. & Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset: A sex-linked recessive trait. Neurology 18, 671–680 (1968).
Olney, R.K., Aminoff, M.J. & So, Y.T. Clinical and electrodiagnostic features of X-linked recessive bulbospinal neuronopathy. Neurology 47, 1111–1120 (1990).
Arbizu, T., Santamaria, J., Gomez, J.M., Quilez, A. & Serra, J.P. A family with adult spinal and bulbar muscular atrophy, X-linked inheritance and associated testicular failure. J. neurol. Sci. 59, 371–382 (1983).
Brown, C.J. et al. Androgen receptor locus on the X chromosome: Regional localization to Xq 11–12 and description of a DNA polymorphism. Am. J. hum. Genet. 44, 264–269 (1989).
Fischbeck, K.H. et al. Localization of the gene for X-linked spinal muscular atrophy. Neurology 36, 1595–1598 (1986).
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Edwards, A., Hammond, H.A., Jin, L., Caskey, C.T. & Chakraborty, R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics 12, 241–253 (1992).
La Spada, A.R. et al. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nature Genet. 2, 301–304 (1992).
The Huntingdon's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Orr, H.T. et al. Expansion of an unstable trinuleotide CAG repeat in spinocerebellar ataxia type 1. Nature Genet. 4, 221–226 (1993).
Doyu, M. et al. Severity of X-linked recessive bulbospinal neuronopathy correlates with size of the tandem CAG repeat in androgen receptor gene. Ann. Neurol. 32, 707–710 (1992).
Biancalana, V. et al. Moderate instability of the trinucleotide repeat in spinobulbar muscular atrophy. Hum. molec. Genet. 1, 255–258 (1992).
Brook, J.D. et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Verkerk, A.J.M.H. et al. Identification of a gene (FMR–1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1992).
Chang, C., Kokontis, J. & Liao, S. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc. natn. Acad. Sci. U.S.A. 85, 7211–7215 (1988).
Faber, P.W. et al. The N-terminal domain of the human androgen receptor is encoded by one, large exon. Molec. cell. Endocrinol. 61, 257–262 (1989).
Faber, P.W. et al. The mouse androgen receptor: functional analysis of the protein and characterization of the gene. Biochem. J. 278, 269–278 (1991).
Kao, C.C. et al. Cloning of a transcriptionally active human TATA binding factor. Science 248, 1646–1649 (1990).
Riggins, G.J. et al. Human genes containing polymorphic trinucleotide repeats. Nature Genet. 2, 186–191 (1992).
Courey, A.J. & Tjian, R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55, 887–898 (1988).
Pirotta, V., Manet, E., Hardon, E., Bickel, S.E. & Benson, M. Structure and sequence of the Drosphilia zeste gene. EMBO J. 6, 791–799 (1987).
Blochlinger, K., Bodmer, R., Jack, J., Jan, J.Y. & Jan, Y.N. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosphilia. Nature 333, 629–635 (1988).
Sar, M. & Stumpf, W.E. Androgen concentration in motor neurons of cranial nerves and spinal cord. Science 197, 77–80 (1977).
Sheridan, P.J. & Weaker, F.J. Androgen receptor systems in the brain stem of the primate. Brain Res. 235, 225–232 (1982).
Yu, W.A. & McGinnis, M.Y. Androgen receptor levels in cranial nerve nuclei and tongue muscles in rats. J. Neurosci 6, 1302–1307 (1986).
Hauser, K.F., MacLusky, N.J. & Toran-Allerand, C.D. Androgen action in fetal mouse spinal cord cultures: Metabolic and morphologic aspects. Brain Res. 406, 62–72 (1987).
Breedlove, S.M. Cellular analyses of hormone influence on motor neuronal development and function. J. Neurobiol. 17, 157–176 (1986).
Yu, W.A. & Yu, M.C. Acceleration of the regeneration of the crushed hypoglossal nerve by testosterone. Exp. Neurol. 80, 349–360 (1983).
Yu, W.A. Administration of testosterone attenuates neuronal loss following axotomy in the brain-stem motor nuclei of female rats. J. Neurosci. 9, 3908–3914 (1989).
Kujawa, K.A., Kinderman, N.B. & Jones, K.J. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp. Neurol. 105, 80–85 (1989).
Goldstein, L.A. & Sengelaub, D.R. Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J. comp. Neurol. 326, 147–157 (1992).
Jenster, G. et al. Domains of the human androgen receptor involved in steroid binding, transcriptional activation and subcellular localization. Molec. Endocrinol. 5, 1396–1404 (1991).
Simental, J.A., Sar, M., Lane, M.V., French, F.S. & Wilson, E.M. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J. biol. Chem. 266, 510–518 (1991).
Tora, L., Gronemeyer, H., Turcotte, B., Gaub, M-P. & Chambon, P., The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature 333, 185–188 (1988).
Tora, L. et al. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59, 477–487 (1989).
Stråhle, U. et al. Cooperative action of the glucocorticoid receptor and transcription factors. Cold Spring Harbor Symposia on Quantitative Biology 53, 835–841 (1988).
Levine, M. & Manley, J.L. Transcriptional repression of eukaryotic promoters. Cell 59, 405–408 (1989).
Yang-Yen, H-S. et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62, 1205–1215 (1990).
Frankel, A.D. & Kim, P.S. Modular structure of transcription factors: implications for gene regulation. Cell 65, 717–719 (1991).
Ing, N.H., Beekman, J.M., Tsai, S.Y., Tsai, M-J. & O'Malley, B.W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300–II). J. biol. Chem. 267, 17617–17623 (1992).
Mhatre, A. et al. Host cell-restricted expression of a ligand-selective mutant androgen receptor phenotype. Am. J. hum. Genet. 51, A321, Abst 1264, 1992.
Miesfield, R. et al. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell 46, 389–399 (1986).
Hollenberg, S.M. et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 318, 635–641 (1985).
Danielson, M., Northrop, J. & Ringold, G. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 5, 2513–2522 (1986).
McPhaul, M.J. et al. Molecular basis of androgen resistance in a family with a qualitative abnormality of the androgen receptor and responsive to high-dose androgen therapy. J. clin. Invest. 87, 1413–1421 (1991).
Warner, C.L. et al. X-linked spinomuscular atrophy: a kindred with associated abnormal androgen receptor binding. Neurology 42, 2181–2184 (1992).
Matsuura, T. et al. Androgen receptor abnormality in X-linked spinal and bulbar muscular atrophy. Neurology 42, 1724–1726 (1992).
Araki, I., Harada, Y. & Kuno, M. Target-dependent hormonal control of neuron size in the rat spinal nucleus of the bulbocavernosus. J. Neurosci. 11, 3025–3033 (1991).
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Higuchi, R. Simple and rapid preparation of samples for PCR, in PCR Technology (ed Erlich H.A.) 31–38 (Stockton Press, New York, 1989).
Brinkmann, A.O. et al. The human androgen receptor: domain structure, genomic organization and regulation of expression. J. steroid Biochem. 34, 307–310 (1989).
MacGregor, G.R. & Caskey, C.T. Construction of plasmids that express E.coli β-galactosidase in mammalian cells. Nucleic Acids Res. 17, 2365 (1989).
Kazemi-Esfarjani, P. et al. Substitution of valine-865 by methionine or leucine in the human androgen receptor causes complete or partial androgen insensitivity, respectively with distinct androgen receptor phenotypes. Molec. Endocrinol. 7, 37–46 (1993).
Gottlieb, B., Kaufman, M., Pinsky, L., Leboeuf, G. & Sotos, J.F. Extracellular correction of the androgen-receptor transformation defect in two families with complete androgen resistance. J. steroid Biochem. 28, 279–284 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mhatre, A., Trifiro, M., Kaufman, M. et al. Reduced transcriptional regulatory competence of the androgen receptor in X–linked spinal and bulbar muscular atrophy. Nat Genet 5, 184–188 (1993). https://doi.org/10.1038/ng1093-184
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1093-184
This article is cited by
-
Investigation of androgen receptor CAG repeats length in polycystic ovary syndrome diagnosed using the new international evidence-based guideline
Journal of Ovarian Research (2023)
-
Androgen receptor with short polyglutamine tract preferably enhances Wnt/β-catenin-mediated prostatic tumorigenesis
Oncogene (2020)
-
MEF2 impairment underlies skeletal muscle atrophy in polyglutamine disease
Acta Neuropathologica (2020)
-
The polyglutamine-expanded androgen receptor responsible for spinal and bulbar muscular atrophy inhibits the APC/CCdh1 ubiquitin ligase complex
Scientific Reports (2016)
-
Genetics of androgen metabolism in women with infertility and hypoandrogenism
Nature Reviews Endocrinology (2015)