Abstract
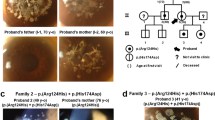
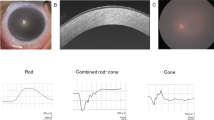
Macular corneal dystrophy (MCD; MIM 217800) is an autosomal recessive hereditary disease in which progressive punctate opacities in the cornea result in bilateral loss of vision, eventually necessitating corneal transplantation. MCD is classified into two subtypes, type I and type II, defined by the respective absence and presence of sulphated keratan sulphate in the patient serum, although both types have clinically indistinguishable phenotypes1,2. The gene responsible for MCD type I has been mapped to chromosome 16q22, and that responsible for MCD type II may involve the same locus3,4,5. Here we identify a new carbohydrate sulphotransferase gene (CHST6), encoding an enzyme designated corneal N-acetylglucosamine-6-sulphotransferase (C-GlcNAc6ST), within the critical region of MCD type I. In MCD type I, we identified several mutations that may lead to inactivation of C-GlcNAc6ST within the coding region of CHST6. In MCD type II, we found large deletions and/or replacements caused by homologous recombination in the upstream region of CHST6. In situ hybridization analysis did not detect CHST6 transcripts in corneal epithelium in an MCD type II patient, suggesting that the mutations found in type II lead to loss of cornea-specific expression of CHST6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yang, C.J., SundarRaj, N., Thonar, E.J. & Klintworth, G.K. Immunohistochemical evidence of heterogeneity in macular corneal dystrophy. Am. J. Ophthalmol. 106, 65–71 (1988).
Edward, D.P. et al. Heterogeneity in macular corneal dystrophy. Arch. Ophthalmol. 106, 1579–1583 (1988).
Vance, J.M. et al. Linkage of a gene for macular corneal dystrophy to chromosome 16. Am. J. Hum. Genet. 58, 757–762 (1996).
Jonasson, F. et al. Macular corneal dystrophy in Iceland. A clinical, genealogic, and immunohistochemical study of 28 patients. Ophthalmology 103, 1111–1117 (1996).
Liu, N.P. et al. Haplotype analysis in Icelandic families defines a minimal interval for the macular corneal dystrophy type I gene. Am. J. Hum. Genet. 63, 912–917 (1998).
Nakazawa, K. et al. Defective processing of keratan sulfate in macular corneal dystrophy. J. Biol. Chem. 259, 13751–13757 (1984).
Klintworth, G.K. et al. Macular corneal dystrophy. Lack of keratan sulfate in serum and cornea. Ophthalmic Paediatr. Genet. 7, 139–143 (1986).
Thonar, E.J. et al. Absence of normal keratan sulfate in the blood of patients with macular corneal dystrophy. Am. J. Ophthalmol. 102, 561–569 (1986).
Edward, D.P., Thonar, E.J., Srinivasan, M., Yue, B.J. & Tso, M.O. Macular dystrophy of the cornea. A systemic disorder of keratan sulfate metabolism. Ophthalmology 97, 1194–1200 (1990).
Altschul, S.F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Lee, J.K., Bhakta, S., Rosen, S.D. & Hemmerich, S. Cloning and characterization of a mammalian N-acetylglucosamine-6-sulfotransferase that is highly restricted to intestinal tissue. Biochem. Biophys. Res. Commun. 263, 543–549 (1999).
Kakuta, Y., Pedersen, L.G., Pedersen, L.C. & Negishi, M. Conserved structural motifs in the sulfotransferase family. Trends Biochem. Sci. 23, 129–130 (1998).
Kakuta, Y., Sueyoshi, T., Negishi, M. & Pedersen, L.C. Crystal structure of the sulfotransferase domain of human heparan sulfate N-deacetylase/N-sulfotransferase 1. J. Biol. Chem. 274, 10673–10676 (1999).
Klintworth, G.K. et al. Macular corneal dystrophy in Saudi Arabia: a study of 56 cases and recognition of a new immunophenotype. Am. J. Ophthalmol. 124, 9–18 (1997).
Liu, N.P. et al. Coexistence of macular corneal dystrophy types I and II in a single sibship. Br. J. Ophthalmol. 82, 241–244 (1998).
Thonar, E.J. et al. Quantification of keratan sulfate in blood as a marker of cartilage catabolism. Arthritis Rheum. 28, 1367–1376 (1985).
Fukuta, M. et al. Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J. Biol. Chem. 272, 32321–32328 (1997).
Uchimura, K. et al. Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis X: molecular cloning, chromosomal mapping, and expression in various organs and tumor cells. J. Biochem. (Tokyo) 124, 670–678 (1998).
Fukuta, M., Kobayashi, Y., Uchimura, K., Kimata, K. & Habuchi, O. Molecular cloning and expression of human chondroitin 6-sulfotransferase. Biochim. Biophys. Acta 1399, 57–61 (1998).
Bistrup, A. et al. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J. Cell Biol. 145, 899–910 (1999).
Kawakami, M. & Nakayama, J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 57, 2321–2324 (1997).
Shiozawa, T., Tsukahara, Y., Nakayama, J., Ishii, K. & Katsuyama, T. Immunohistochemical reactivity of antikeratan sulfate monoclonal antibody 5D4 to various conditions of human endometrial tissues and its application as a useful marker for identifying endometrial epithelia. Gynecol. Obstet. Invest. 32, 239–242 (1991).
Hudson, T.J., Clark, C.D., Gschwend, M. & Justice-Higgins, E. PCR methods of genotyping. in Current Protocols in Human Genetics (eds Dracopoli, N.C. et al.) 2.5.1–2.5.23 (John Wiley & Sons, New York, 1997).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Kakuta, Y., Pedersen, L.G., Carter, C.W., Negishi, M. & Pedersen, L.C. Crystal structure of estrogen sulphotransferase. Nature Struct. Biol. 4, 904–908 (1997).
Acknowledgements
We thank N. Hiraoka, R. Aoki, H. Nakagawa, S. Saburi and A. Suzuki for discussions; C. Sotozono for patient DNA samples; M. Onda for normal Japanese samples; and S. Kubota and A. Pai for technical assistance. This work was supported by NIH grant CA71932 (M.N.F.), AG04736 and AR39239 (E.J.-M.A.T.). This work was also supported by Grant-in-Aid for Scientific Research (09671800, 10178104, 10470365) from the Ministry of Education, Science, Sports and Culture of Japan (H.W., J.N. and S.K.)
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Rights and permissions
About this article
Cite this article
Akama, T., Nishida, K., Nakayama, J. et al. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet 26, 237–241 (2000). https://doi.org/10.1038/79987
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/79987
This article is cited by
-
The role of corneal endothelium in macular corneal dystrophy development and recurrence
Science China Life Sciences (2024)
-
Endoplasmic reticulum stress: molecular mechanism and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Macular corneal dystrophy related to novel mutations of CHST6 in a Chinese family and clinical observation after penetrating keratoplasty
BMC Medical Genomics (2021)
-
Detailed corneal and genetic characteristics of a pediatric patient with macular corneal dystrophy - case report
BMC Ophthalmology (2021)
-
Impairment of the autophagy-lysosomal pathway and activation of pyroptosis in macular corneal dystrophy
Cell Death Discovery (2020)