Abstract
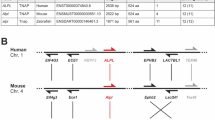
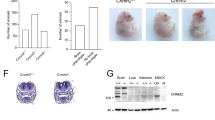
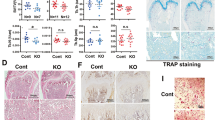
In humans, deficiency of the tissue non-specific alkaline phosphatase (TNAP) gene is associated with defective skeletal mineralization. In contrast, mice lacking TNAP generated by homologous recombination using embryonic stem (ES) cells have normal skeletal development. However, at approximately two weeks after birth, homozygous mutant mice develop seizures which are subsequently fatal. Defective metabolism of pyridoxal 5′-phosphate (PLP), characterized by elevated serum PLP levels, results in reduced levels of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the brain. The mutant seizure phenotype can be rescued by the administration of pyridoxal and a semi-solid diet. Rescued animals subsequently develop defective dentition. This study reveals essential physiological functions of TNAP in the mouse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
McComb, R.B., Bowers Jr, G.N. & Posen, S. Alkaline Phosphatase (Plenum Press, New York, NY, 1979).
Low, M.G. & Saltiel, A.R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science 239, 268–275 (1988).
Henthom, P.S., Raducha, M., Kadesch, T., Weiss, M.J. & Harris, H. Sequence and characterization of the human intestinal alkaline phosphatase gene. J. biol. Chem. 263, 12011–12019 (1988).
Knoll, B.J., Rothblum, K.N. & Longley, M. Nucleotide sequence of the human placental alkaline phosphatase gene. Evolution of the 5′ flanking region by deletion/substitution J. biol. Chem. 263, 12020–12027 (1988).
Millán, J.L. & Manes, T. Seminoma-derived Nagao isozyme is encoded by a germ-cell alkaline phosphatase gene. Proc. natn. Acad. Sci. U.S.A. 85, 3024–3028 (1988).
Weiss, M.J. et al. Structure of the human liver/bone/kidney alkaline phosphatase gene. J. biol. Chem. 263, 12002–12010 (1988).
Manes, T., Glade, K., Ziomek, C.A. & Millán, J.L. Genomic structure and comparison of mouse tissue-specific alkaline phosphatase genes. Genomics 8, 541–554 (1990).
Hahnel, A.C. et al. Two alkaline phosphatase genes are expressed during early development in the mouse embryo. Development 110, 555–564 (1990).
Hahnel, A.C. & Schultz, G.A. Cloning and characterization of a cDNA encoding alkaline phosphatase in mouse embryonal carcinoma cells. Clinica Chim. Acta 186, 171–174 (1989).
MacGregor, G.R., Zambrowicz, B.P. & Soriano, P. Tissue non-specific alkaline phosphatase is expressed in both embryonic and extra-embryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development 121, 1487–1496 (1995).
Terao, M. & Mintz, B. Cloning and characterisation of a cDNA coding for mouse placental alkaline phosphatase. Proc. natn. Acad. Sci. U.S.A. 84, 7051–7055 (1987).
Henthorn, P.S., Raducha, M., Fedde, K.N., Lafferty, M.A. & Whyte, M.P. Different missense mutations at the tissue non-specific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc. natn. Acad. Sci. U.S.A. 89, 9924–9928 (1992).
Whyte, M.P. in The Metabolic Basis of Inherited Disease, 7th Edition. (eds Scriver, C.R., Beaudet, A.L., Sly, W.S. & Valle, D.) 4095–4111 (McGraw-Hill, New York, 1995).
Whyte, M.P., Teitelbaum, S.L., Murphy, W.A., Bergfeld, M.A. & Avioli, L.V. Adult hypophosphatasia: clinical, laboratory and genetic investigation of a large kindred with review of the literature. Medicine 58, 329–347 (1979).
Whyte, M.P., Murphy, W.M. & Fallen, M.D. Adult hypophosphatasia with chondrocalcinosis and arthropathy : Variable penetrance of hypophosphatasemia in a large Oklahoma kindred. Am. J. Med. 72, 631–641 (1982).
Macfarlane, J.D., Kroon, H.M. & van der Harten, J.J. Phenotypically dissimilar hypophosphatasia in two sibships. Am. J. med. Genet. 42, 117–121 (1992).
Ritchie, G.M. Hypophosphatasia: a metabolic disease with important dental manifestations. Arch. Dis. Child. 39, 584–590 (1964).
Houpt, M.I., Kenny, F.M. & Listgarten, M. Hypophosphatasia: case reports. J. Dent Child. 27, 126–137 (1970).
Bruckner, R.J., Rickles, N.H. & Porter, D.R. Hypophosphatasia with premature shedding of teeth and aplasia of cementum. Oral Path. 15, 1351–1369 (1962).
Rasmussen, K. Phosphorylethanolamine and hypophosphatasia. Danish Medical Bulletin 15, 1–112 (1968).
Whyte, M.P. et al. Perinatal hypophosphatasia: tissue levels of vitamin B-6 are unremarkable despite markedly increased circulating concentrations of pyridoxal-5′-phosphate. J. Clin. Invest. 81, 1234–1239 (1988).
Takahashi, T. et al. in Endocrine control of bone and calcium metabolism. (eds Chon, D.V., Fugita, T., Potts, J.T.J. & Talmage, R.V.) 93–94 (Excerpta Medica, Amsterdam, 1984).
Roxburgh, S.T.D. Atypical retinitis pigmentosa with hypophosphatasia. Trans. ophthal. Soc. U.K. 103, 513–516 (1983).
Warshaw, J.B., Littlefield, J.W., Fishman, W.H., Inglis, N.R. & Stolbach, L.L. Serum alkaline phosphate in hypophosphatasia. J. din. Invest. 50, 2137–2142 (1971).
Ornoy, A., Adomian, G.E. & Rimoin, D.L. Histologic and ultrastructural studies on the mineralisation process in hypophosphatasia. Am. J. Med. Genet. 22, 743–758 (1985).
Whyte, M.P., Mahuren, J.D., Vrabel, L.A. & Cobum, S.P. Markedly increased circulating pyridoxal 5′-phosphate levels in hypophosphatasia. J. Clin. Invest. 76, 752–756 (1985).
Snell, E.E. & Haskell, B.E. in Comprehensive Biochemistry. (eds Florkin, M. & Stotz, E.H.) 47–71 (Elsevier, Amsterdam, 1971).
Fedde, K.N., Cole, D.E. & Whyte, M.P. Pseudohypophosphatasia: aberrant localization and substrate specificity of alkaline phosphatase in cultured skin fibrobiasts. Am. J. hum. Genet. 47, 776–783 (1990).
Fedde, K.N. & Whyte, M.P. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal 5′-phosphate ectophosphatase: normal and hypophosphatasia fibroblast study. Am. J. hum. Genet. 47, 767–775 (1990).
Fonda, M.L., Eggers, D.K., Auerbach, S. & Fritsch, L. Vitamin B-6 metabolism in the brains of young adult and senescent mice. Exp. Geront. 15, 473–479 (1980).
Bukin, Y.V. Pyridoxal kinase activity and content of pyridoxal phosphate in mammalian tissues under normal and some experimental conditions. Biokhimiya 41, 81–90 (1976).
Shideler, C.E. Vitamin B-6: an overview. Am. J. med. Tech. 49, 17–22 (1983).
Voet, D. & Voet, J.G. Biochemistry. (Wiley, New York, 1990).
Meldrum, B.S. Epilepsy and γ-aminobutyric acid-mediated inhibition. Int. Rev. Neurobiol. 17, 1–36 (1975).
Dakshinamurti, K. & Stephens, M.C. Pyridoxine deficiency in the neonatal rat. J. Neurochem. 16, 1515–1522 (1969).
Stephens, M.C., Havlicek, V. & Dakshinamurti, K. Pyridoxine deficiency and development of the central nervous system in the rat. J. Neurochem. 18, 2407–2416 (1971).
Guilarte, T.R. Regional changes in the concentrations of glutamate, glycine, taurine and GABA in the vitamin B-6 deficient developing rat brain: association with neo-natal seizures. Neurochem. Res. 14, 889–897 (1989).
Wasynczuk, A., Kirksey, A. & Morre, D.M. Effects of maternal vitamin B-6 deficiency on specific regions of developing rat brain : the extrapyramidal motor system. J. Nutrition 113, 746–754 (1983).
Ebadi, M. Regulation and function of pyridoxal phosphate in CNS. Neurochem. Intl. 3, 181–206 (1981).
Bayoumi, R.A. & Smith, W.R.D. Someeffectsof dietary vitamin B-6 deficiency on γaminobutyric acid metabolism in developing rat brain. J. Neurochem. 19, 1883–1897 (1972).
Eastman, C.L. & Guilarte, T.R. Vitamin B-6, kynurenines and central nervous system function : developmental aspects. J. Nutr. Biochem. 3, 618–631 (1992).
Caswell, A.M., Whyte, M.P. & Russell, R.G.G. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: clinical and laboratory aspects. Crit. Rev. din. Lab. Sci. 28, 175–232 (1991).
Wada, H. & Snell, E.E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J. biol. Chem. 236, 2089–2095 (1961).
Krinke, G., Naylor, D.C. & Skorpil, V. Pyridoxine vitaminosis: an analysis of the early changes induced with massive doses of vitamin B-6 in rat primary sensory neurons. J. Neuropathol. exp. Neurol. 44, 117–129 (1985).
Krinke, G. et al. Pyridoxine megavitaminosis produces degeneration of peripheral sensory neurons in the dog. Neurotoxicology 2, 13–24 (1980).
Albin, R.L. et al. Acute sensory neuropathy—neuronopathy from pyridoxine overdose. Neurology 37, 1729–1732 (1987).
Salhany, J.M. & Schopfer, L.M. Pyridoxal 5′-phosphate binds specifically to soluble CD4 protein, the HIV-1 receptor. J. biol. Chem. 268, 7643–7645 (1993).
Salhany, J.M., Rauenbuehler, P.B. & Sloan, R.L. Characterisation of pyridoxal 5′-phosphate affinity labelling of band 3 protein. J. biol. Chem. 262, 15965–15973 (1987).
Langman, M.J.S. et al. Influence of diet on the intestinal component of serum alkaline phosphatase in people of different ABO blood groups and secretor status. Nature 212, 41–43 (1966).
Gould, B.S. The nature of the increased serum phosphatase in rats after fat feeding. Arch. Biochem. 4, 175–181 (1944).
Whyte, M.P. et al. Alkaline phosphatase: placental and tissue-nonspecific isoenzymes hydrolyse phosphoethanolamine, inorganic pyrophosphate and pyridoxal 5′-phosphate. J. Clin. Invest. 95, 1440–1445 (1995).
Friedrich, G.A. & Soriano, P. Promoter traps in embryonic stem cells: genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 (1990).
Slocum, R.H. & Cummings, J.G. in Techniques in diagnostic human biochemical genetics: a laboratory manual. (ed. Hommes, F.A.) 87–128 (Wiley-Liss, 1991).
Mahuren, J.D. & Cobum, S.P. B-6 vitamers: cation exchange HPLC. Nutr. Biochem. 1, 659–663 (1990).
Bell, R.R. & Haskell, B.E. Metabolism of vitamin B-6 in the l-strain mouse. Arch. Biochem. Biophys. 147, 588–601 (1971).
Sasaki, T. Cell biology of tooth enamel formation. 1–204 (Karger, Basel, 1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Waymire, K., Mahuren, J., Jaje, J. et al. Mice lacking tissue non–specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B–6. Nat Genet 11, 45–51 (1995). https://doi.org/10.1038/ng0995-45
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0995-45
This article is cited by
-
The diagnosis of hypophosphatasia in children as a multidisciplinary effort: an expert opinion
Journal of Endocrinological Investigation (2023)
-
Monitoring protein conformational changes using fluorescent nanoantennas
Nature Methods (2022)
-
A new perspective on the function of Tissue Non-Specific Alkaline Phosphatase: from bone mineralization to intra-cellular lipid accumulation
Molecular and Cellular Biochemistry (2022)
-
GSK3β rephosphorylation rescues ALPL deficiency-induced impairment of odontoblastic differentiation of DPSCs
Stem Cell Research & Therapy (2021)
-
Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice
Communications Biology (2021)