Abstract
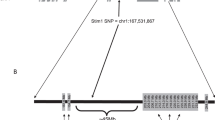
Ischaemic stroke is a complex disorder caused by a combination of genetic and environmental factors. Clinical and epidemiological studies have provided strong evidence for genetic influences in the development of human stroke1–3 and several mendelian traits featuring stroke have been described4,5. The genetic analysis of the non-mendelian, common ischaemic stroke in humans is hindered by the late onset of the disease and the mode of inheritance, which is complex, polygenic and multifactorial. An important approach to the study of such polygenic diseases is the use of appropriate animal models in which individual contributing factors can be recognized and analysed6,7. The spontaneously hypertensive stroke-prone rat (SHRSP) is an experimental model of stroke characterized by a high frequency of spontaneous strokes8 as well as an increased sensitivity to experimentally induced focal cerebral ischaemia9,10. Rubattu et al.11 performed a genome-wide screen in an F2 cross obtained by mating SHRSP and SHR, in which latency to stroke on Japanese rat diet was used as a phenotype. This study identified three major quantitative trait loci (QTLs), STR-1–3. Of these, STIR-2 and 3 conferred a protective effect against stroke in the presence of SHRSP alleles and STR-2 co-localized with the genes encoding for atrial natriuretic and brain natriuretic factors11. Our investigation was designed to identify the genetic component responsible for large infarct volumes in the SHRSP in response to a focal ischaemic insult by performance of a genome scan in an F2 cross derived from the SHRSP and the normotensive reference strain, WKY rat. We identified a highly significant QTL on rat chromosome 5 with a lod score of 16.6 which accounts for 67% of the total variance, co-localizes with the genes encoding atrial and brain natriuretic factor and is blood pressure independent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Alberts, M.J. Genetic aspects of cerebrovascular disease. Stroke 21, 276–280 (1991).
Kiely, D.K., Wolf, P.A., Cupples, A., Beiser, A.S. & Meyers, R.H. Familial aggregation of stroke: the Framingham Study. Stroke. 24, 1366–1371 (1993).
Brass, L.M., Isaacsohn, J.L., Merikangas, K.R. & Robinette, C.D. A study of twins and stroke.Stroke 23, 221–223 (1992).
Gunel, M. & Lifton, R.P. Counting strokes. Nature Genet. 13, 384–385 (1996).
Joutel, A. et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature Genet. 383, 707–710 (1996).
Lander, E.S. & Schork, N.J. Genetic dissection of complex traits. Science 265, 2037–2048 (1994).
Dominiczak, A.F. & Lindpaintner, K. Genetics of hypertension: a current appraisal. News Physiol Sci. 9, 246–251 (1994).
Okamoto, K., Yamori, Y. & Nagaoka, A. Establishment of the stroke-prone spontaneously hypertensive rat (SHR). Circ. Res. 34-45, (Suppl) 142–153 (1974).
Coyle, P. & Jokelainen, P.T. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar-Kyoto (WKY) rats. Stroke 14, 605–611 (1983).
Coyle, P., Odenheimer, D.J. & Sing, C.F. Cerebral infarction after middle cerebral artery occlusion in progenies of spontaneously stroke-prone and normal rats. Stroke 15, 711–716 (1984).
Rubattu, S. et al. Chromosomal mapping of quantitative trait loci contributing to stroke in a rat model of complex human disease. Nature Genet. 13, 429–434 (1996).
Clark, J.S. et al. Quantitiative trait loci in genetically hypertensive rats: possible sex specificity. Hypertension 28, 898–906 (1996).
Davidson, A.O. et al. Blood pressure in genetically hypertensive rats: influence of the Y chromosome. Hypertension 26, 452–459 (1995).
Coyle, P. & Heistad, D.D. Development of collaterals in the cerebral circulation. Blood Vessels 28, 183–189 (1991).
Arribas, A.M., Gordon, J.F., Daly, C.J., Dominiczak, A.F. & McGrath, J.C. Confocal microscopic characterization of a lesion in a cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke 27, 1118–1123 (1996).
Volpe, M. et al. Association and cosegregation of stroke with impaired endothelium-dependent vasorelaxation in stroke prone, spontaneously hypertensive rats. J. Clin. Invest. 98, 256–261 (1996).
Quirion, R., Dalpe, M. & Dam, T.V. Characterization and distribution of receptors for the atrial natriuretic peptides in mammalian brain. Proc. Natl. Acad. Sci. USA 83, 174–178 (1986).
Estrada, V. et al. High plasma levels of endothelin-1 and atrial natriuretic peptide in patients with acute ischemic stroke. Am. J. Hypertens. 7, 1085–1089 (1994).
Jacewicz, M. The hypertensive rat and predisposition to cerebral infarction. Hypertension 19, 47–48 (1992).
Dominiczak, A.F. et al. Lateral diffusion and fatty acid composition in vascular smooth muscle membrane from stroke-prone spontaneously hypertensive rats. Am. J. Hypertens. 6, 1003–1008 (1993).
Tamura, A., Graham, D.I., McCulloch, J. & Teasdale, G.M. Focal ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab 1, 53–60 (1981).
Konig, J.F.R. & Klippel, R.A., The Rat Brain: A Stereotaxic Atlas of the Forebrain and Lower parts of the Brain Stem. (Krieger, New York, 1963).
Osborne, K.A. et al. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J. Neurol. Neurosurg. Psychiatry 50, 402–410 (1987).
Jacob, H.J. et al. A genetic linkage map of the laboratory rat, Rattus norvegicus. Nature Genet. 9, 63–69 (1995).
Lander, E.S. et al. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1, 174–181 (1987).
Lander, E.S. & Botstein, D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121, 185–189 (1989).
Rawlings, J.O., Applied Regression Analysis a Research Tool(Wadsworth and Brodes/Cole Publishing Company, Pacific Grove, California, 1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeffs, B., Clark, J., Anderson, N. et al. Sensitivity to cerebral ischaemic insult in a rat model of stroke is determined by a single genetic locus. Nat Genet 16, 364–367 (1997). https://doi.org/10.1038/ng0897-364
Issue Date:
DOI: https://doi.org/10.1038/ng0897-364
This article is cited by
-
HDAC9 Polymorphisms Predict Susceptibility, Severity, and Short-Term Outcome of Large Artery Atherosclerotic Stroke in Chinese Population
Journal of Molecular Neuroscience (2019)
-
Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment
Molecular Neurodegeneration (2015)
-
Association between paraoxonase gene and stroke in the Han Chinese population
BMC Medical Genetics (2013)
-
Standards and pitfalls of focal ischemia models in spontaneously hypertensive rats: With a systematic review of recent articles
Journal of Translational Medicine (2012)
-
The stroke-prone spontaneously hypertensive rat: still a useful model for post-GWAS genetic studies?
Hypertension Research (2012)