Abstract
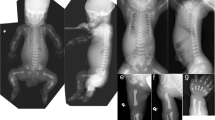
Robinow syndrome is a short-limbed dwarfism characterized by abnormal morphogenesis of the face and external genitalia, and vertebral segmentation1,2. The recessive form of Robinow syndrome (RRS; OMIM 268310), particularly frequent in Turkey3,4,5,6, has a high incidence of abnormalities of the vertebral column such as hemivertebrae and rib fusions, which is not seen in the dominant form. Some patients have cardiac malformations or facial clefting. We have mapped a gene for RRS to 9q21–q23 in 11 families. Haplotype sharing was observed between three families from Turkey, which localized the gene to a 4.9-cM interval. The gene ROR2, which encodes an orphan membrane-bound tyrosine kinase, maps to this region. Heterozygous (presumed gain of function) mutations in ROR2 were previously shown to cause dominant brachydactyly type B (BDB; ref. 7). In contrast, Ror2−/− mice have a short-limbed phenotype that is more reminiscent of the mesomelic shortening observed in RRS. We detected several homozygous ROR2 mutations in our cohort of RRS patients that are located upstream from those previously found in BDB. The ROR2 mutations present in RRS result in premature stop codons and predict nonfunctional proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Robinow, M., Silverman, F.N. & Smith, H.D. A newly recognised dwarfing syndrome. Am. J. Dis. Child. 117, 645–651 (1969).
Butler, M.G. & Wadlington, W.B. Robinow syndrome: reports of two patients and review of literature. Clin. Genet. 31, 77–85 (1987).
Balci, S., Beksac, S., Haliloglu, M., Ercis, M. & Eryilmaz, M. Robinow syndrome, vaginal atresia, hematocolpos, and extra middle finger. Am. J. Med. Genet. 79, 27–29 (1998).
Balci, D., Ercal, M.D., Say, B. & Atasu, M. Robinow syndrome: with special emphasis on dermatoglyphics and hand malformations (split hand). Clin. Dysmorphol. 2, 199–207 (1993).
Aksit, S. et al. Is the frequency of Robinow syndrome relatively high in Turkey? Four more case reports. Clin. Genet. 52, 226–230 (1997).
Atalay, S. et al. Congenital heart disease and Robinow syndrome. Clin. Dysmorphol. 2, 208–210 (1993).
Oldridge, M. et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nature Genet. 24, 275–278 (2000).
DeChiara, T.M. et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nature Genet. 24, 271–274 (2000).
Takeuchi, S. et al. Mouse ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 5, 71–78 (2000).
Oishi, I. et al. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells 4, 41–56 (1999).
Masiakowski, P. & Carroll, R.D. A novel family of cell surface receptor with tyrosine kinase-like domain. J. Biol. Chem. 267, 26181–26190 (1992).
Wilson, C., Goberdhan, D.C.I. & Steller, H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor tyrosine kinases. Proc. Natl Acad. Sci. USA 90, 7109–7113 (1993).
Oishi, I. et al. A novel Drosophila receptor tyrosine kinase expressed specifically in the nervous system. Unique structural features and implication in developmental signaling. J. Biol. Chem. 272, 11916–11923 (1997).
Forrester, W.C., Dell, M., Perens, E. & Garriga, G. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature 400, 881–885 (1999).
Furie, B. & Furie, B.C. The molecular basis of blood coagulation. Cell 53, 505–518 (1988).
Nakamura, T. et al. Molecular cloning and expression of human hepatocyte growth factor. Nature 342, 440–443 (1989).
Cunningham, M.E., Stephens, R.M., Kaplan, D.R. & Greene, L.A. Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J. Biol. Chem. 272, 10957–10967 (1997).
Hanks, S.K. & Quinn, A.M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200, 38–62 (1991).
Obermeier, A. et al. Tyrosine 785 is a major determinant of Trk-substrate interaction. EMBO J. 12, 933–941 (1993).
Loeb, D.M., Stephens, R.M., Copeland, T., Kaplan, D.R. & Greene, L.A. A Trk nerve growth factor (NGF) receptor point mutation affecting interaction with phospholipase C-g1 abolishes NGF-promoted peripherin induction but not neurite outgrowth. J. Biol. Chem. 269, 8901–8910 (1994).
Wilkie, A.O.W. Craniosynostosis: genes and mechanisms. Hum. Mol. Genet. 6, 1647–1656 (1997).
Shiang, R. et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78, 335–342 (1994).
Tavormina, P.L. et al. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nature Genet. 9, 321–328 (1995).
Bellus, G.A. et al. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nature Genet. 14, 174–176 (1996).
Tavormina, P.L. et al. A novel skeletal dysplasia with developmental delay and acanthosis nigricans is caused by a lys650-to-met mutation in the fibroblast growth factor receptor 3 gene. Am. J. Hum. Genet. 64, 722–731 (1999).
Li, C. et al. A Lys644Glu substitution in fibroblast growth factor receptor 3 (FGFR3) causes dwarfism in mice by activation of STATs and ink4 cell cycle inhibitors. Hum. Mol. Genet. 8, 35–44 (1999).
Deng, C., Wynshaw-Boris, A., Zhou, F., Kuo, A. & Leder, P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84, 911–921 (1996).
Kremer, H. et al. Localization of the gene for dominant cystoid macular dystrophy on chromosome 7p. Hum. Mol. Genet. 3, 299–302 (1994).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380, 152–154 (1996).
Lathrop, G.M. & Lalouel, J.M. Easy calculations of LOD scores and genetic risks on small computers. Am. J. Hum. Genet. 36, 460–465 (1984).
Acknowledgements
We thank E. Bosgoed for technical assistance; M.A.M. van Steensel for discussions; and A. Wilkie for sharing genomic sequence data for ROR2. This work was supported by grants from the Dutch Foundation for Scientific Research (NWO) and by the Jan Dekker/Lutgardine Bouwman stichting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Bokhoven, H., Celli, J., Kayserili, H. et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet 25, 423–426 (2000). https://doi.org/10.1038/78113
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78113
This article is cited by
-
Role of the Ror family receptors in Wnt5a signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Planar cell polarity pathway in kidney development, function and disease
Nature Reviews Nephrology (2021)
-
Robinow syndrome in a newborn presenting with hydrocephalus and craniosynostosis
Child's Nervous System (2021)
-
Prospects for pharmacological targeting of pseudokinases
Nature Reviews Drug Discovery (2019)
-
Molecular, functional, and gene expression analysis of zebrafish Ror1 receptor
Fish Physiology and Biochemistry (2019)