Abstract
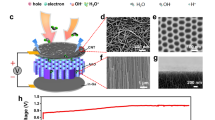
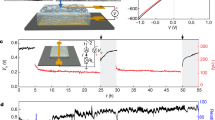
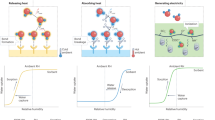
Low-grade heat from sources below 100 ∘C offers a vast quantity of energy. The ability to extract this energy, however, is limited with existing technologies as they are not well-suited to harvest energy from sources with variable heat output or with a small temperature difference between the source and the environment. Here, we present a process for extracting energy from low-grade heat sources utilizing hydrophobic, nanoporous membranes that trap air within their pores when submerged in a liquid. By driving a thermo-osmotic vapour flux across the membrane from a hot reservoir to a pressurized cold reservoir, heat energy can be converted to mechanical work. We demonstrate operation of air-trapping membranes under hydraulic pressures up to 13 bar, show that power densities as high as 3.53 ± 0.29 W m−2 are achievable with a 60 ∘C heat source and a 20 ∘C heat sink, and estimate the efficiency of a full-scale system. The results demonstrate a promising process to harvest energy from low-temperature differences (<40 ∘C) and fluctuating heat sources.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Chen, H., Goswami, D. Y. & Stefanakos, E. K. A review of thermodynamic cycles and working fluids for the conversion of low-grade heat. Renew. Sustain. Energy Rev. 14, 3059–3067 (2010).
Gingerich, D. B. & Mauter, M. S. Quantity, quality, and availability of waste heat from United States thermal power generation. Environ. Sci. Technol. 49, 8297–8306 (2015).
Hentricks, T. & Choate, W. T. Engineering Scoping Study of Thermoelectric Generator Systems for Industrial Waste Heat Recovery (U.S. Department of Energy, 2006).
Blackwell, D. et al. Temperature-at-depth maps for the conterminous U.S. and geothermal resource estimates. Geotherm. Resour. Counc. Trans. 35, 1545–1550 (2011).
Mills, D. Advances in solar thermal electricity technology. Sol. Energy 76, 19–31 (2004).
Barbier, E. Geothermal energy technology and current status: an overview. Renew. Sustain. Energy Rev. 6, 3–65 (2002).
Tchanche, B. F., Lambrinos, G., Frangoudakis, A. & Papadakis, G. Low-grade heat conversion into power using organic Rankine cycles—a review of various applications. Renew. Sustain. Energy Rev. 15, 3963–3979 (2011).
Hung, T. C., Shai, T. Y. & Wang, S. K. A review of organic Rankine cycles (ORCs) for the recovery of low-grade waste heat. Energy 22, 661–667 (1997).
Vélez, F. et al. A technical, economical and market review of organic Rankine cycles for the conversion of low-grade heat for power generation. Renew. Sustain. Energy Rev. 16, 4175–4189 (2012).
Bell, L. E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 321, 1457–1461 (2008).
Zebarjadi, M., Esfarjani, K., Dresselhaus, M. S., Ren, Z. F. & Chen, G. Perspectives on thermoelectrics: from fundamentals to device applications. Energy Environ. Sci. 5, 5147–5162 (2012).
Zhang, F., Liu, J., Yang, W. & Logan, B. E. A thermally regenerative ammonia-based battery for efficient harvesting of low-grade thermal energy as electrical power. Energy Environ. Sci. 8, 343–349 (2015).
Zhang, F., LaBarge, N., Yang, W., Liu, J. & Logan, B. E. Enhancing low-grade thermal energy recovery in a thermally regenerative ammonia battery using elevated temperatures. ChemSusChem 8, 1043–1048 (2015).
Peljo, P., Lloyd, D., Doan, N., Majaneva, M. & Kontturi, K. Towards a thermally regenerative all-copper redox flow battery. Phys. Chem. Chem. Phys. 16, 2831–2835 (2014).
Hu, R. et al. Harvesting waste thermal energy using a carbon-nanotube-based thermo-electrochemical cell. Nano Lett. 10, 838–846 (2010).
Lee, S. W. et al. An electrochemical system for efficiently harvesting low-grade heat energy. Nature Commun. 5, 3942 (2014).
Abraham, T. J., MacFarlane, D. R. & Pringle, J. M. High Seebeck coefficient redox ionic liquid electrolytes for thermal energy harvesting. Energy Environ. Sci. 6, 2639–2645 (2013).
Bocquet, L. Nanofluidics: bubbles as osmotic membranes. Nature Nanotech. 9, 249–251 (2014).
Alkhudhiri, A., Darwish, N. & Hilal, N. Membrane distillation: a comprehensive review. Desalination 287, 2–18 (2012).
Mengual, J. I., Ortiz de Zárate, J., Peña, L. & Velázquez, A. Osmotic distillation through porous hydrophobic membranes. J. Membr. Sci. 82, 129–140 (1993).
Lee, J., Laoui, T. & Karnik, R. Nanofluidic transport governed by the liquid/vapour interface. Nature Nanotech. 9, 317–323 (2014).
El-Bourawi, M. S., Ding, Z., Ma, R. & Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 285, 4–29 (2006).
Cath, T. Y., Adams, V. D. & Childress, A. E. Experimental study of desalination using direct contact membrane distillation: a new approach to flux enhancement. J. Membr. Sci. 228, 5–16 (2004).
Dariel, M. & Kedem, O. Thermoosmosis in semipermeable membranes. J. Phys. Chem. 79, 336–342 (1975).
Mengual, J. I. & Aguilar, J. Thermoosmosis of water through cellulose acetate membranes. J. Membr. Sci. 4, 209–219 (1978).
Tasaka, M., Mizuta, T. & Sekiguchi, O. Mass transfer through polymer membranes due to a temperature gradient. J. Membr. Sci. 54, 191–204 (1990).
Kim, S. & Mench, M. M. Investigation of temperature-driven water transport in polymer electrolyte fuel cell: thermo-osmosis in membranes. J. Membr. Sci. 328, 113–120 (2009).
Adamson, A. W. & Gast, A. P. Physical Chemistry of Surfaces (John Wiley, 1997).
Lee, J. & Karnik, R. Desalination of water by vapor-phase transport through hydrophobic nanopores. J. Appl. Phys. 108, 044315 (2010).
Schofield, R. W., Fane, A. G. & Fell, C. J. D. Heat and mass transfer in membrane distillation. J. Membr. Sci. 33, 299–313 (1987).
Khayet, M. Membranes and theoretical modeling of membrane distillation: a review. Adv. Colloid Interface Sci. 164, 56–88 (2011).
Cusick, R. D., Kim, Y. & Logan, B. E. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science 335, 1474–1477 (2012).
Ramon, G. Z., Feinberg, B. J. & Hoek, E. M. V. Membrane-based production of salinity-gradient power. Energy Environ. Sci. 4, 4423–4434 (2011).
Helfer, F., Lemckert, C. & Anissimov, Y. G. Osmotic power with pressure retarded osmosis: theory, performance and trends—a review. J. Membr. Sci. 453, 337–358 (2014).
Carman, P. C. Flow of Gases Through Porous Media (Academic, 1956).
Kast, W. & Hohenthanner, C. R. Mass transfer within the gas-phase of porous media. Int. J. Heat Mass Transfer 43, 807–823 (2000).
Matsuura, T. Synthetic Membranes and Membrane Separation Processes (CRC Press, 1994).
García-Payo, M., Izquierdo-Gil, M. & Fernández-Pineda, C. Wetting study of hydrophobic membranes via liquid entry pressure measurements with aqueous alcohol solutions. J. Colloid Interface Sci. 230, 420–431 (2000).
Lin, S., Yip, N. Y. & Elimelech, M. Direct contact membrane distillation with heat recovery: thermodynamic insights from module scale modeling. J. Membr. Sci. 453, 498–515 (2014).
Lin, S., Yip, N. Y., Cath, T. Y., Osuji, C. O. & Elimelech, M. Hybrid pressure retarded osmosis—membrane distillation system for power generation from low-grade heat: thermodynamic analysis and energy efficiency. Environ. Sci. Technol. 48, 5306–5313 (2014).
McGinnis, R. L., McCutcheon, J. R. & Elimelech, M. A novel ammonia–carbon dioxide osmotic heat engine for power generation. J. Membr. Sci. 305, 13–19 (2007).
Straub, A. P., Osuji, C. O., Cath, T. Y. & Elimelech, M. Selectivity and mass transfer limitations in pressure-retarded osmosis at high concentrations and increased operating pressures. Environ. Sci. Technol. 49, 12551–12559 (2015).
Qtaishat, M., Matsuura, T., Kruczek, B. & Khayet, A. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 219, 272–292 (2008).
Phattaranawik, J., Jiraratananon, R. & Fane, A. G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 212, 177–193 (2003).
Khayet, M. & Matsuura, T. Preparation and characterization of polyvinylidene fluoride membranes for membrane distillation. Ind. Eng. Chem. Res. 40, 5710–5718 (2001).
Acknowledgements
We acknowledge the National Science Foundation Graduate Research Fellowship DGE-1122492 awarded to A.P.S.
Author information
Authors and Affiliations
Contributions
A.P.S., N.Y.Y., S.L., J.L. and M.E. participated in developing the process and designing the experiments. A.P.S. performed the experiments and analysed the data. A.P.S. and S.L. conducted the system modelling. A.P.S. and M.E. co-wrote the paper. All authors contributed to data analysis, discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Supplementary Figures 1–23, Supplementary Tables 1, Supplementary References. (PDF 1081 kb)
Rights and permissions
About this article
Cite this article
Straub, A., Yip, N., Lin, S. et al. Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nat Energy 1, 16090 (2016). https://doi.org/10.1038/nenergy.2016.90
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.90
This article is cited by
-
Thermal-mechanical-electrical energy conversion system based on Curie effect and soft-contact rotary triboelectric nanogenerator
Nano Research (2023)
-
Roles of thermal energy storage technology for carbon neutrality
Carbon Neutrality (2023)
-
Techno-economic analysis of converting low-grade heat into electricity and hydrogen
Carbon Neutrality (2023)
-
A membrane-based seawater electrolyser for hydrogen generation
Nature (2022)
-
Anomalous thermo-osmotic conversion performance of ionic covalent-organic-framework membranes in response to charge variations
Nature Communications (2022)