Abstract
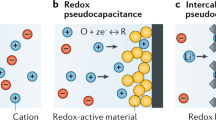
Supercapacitors are electrochemical energy storage devices that operate on the simple mechanism of adsorption of ions from an electrolyte on a high-surface-area electrode. Over the past decade, the performance of supercapacitors has greatly improved, as electrode materials have been tuned at the nanoscale and electrolytes have gained an active role, enabling more efficient storage mechanisms. In porous carbon materials with subnanometre pores, the desolvation of the ions leads to surprisingly high capacitances. Oxide materials store charge by surface redox reactions, leading to the pseudocapacitive effect. Understanding the physical mechanisms underlying charge storage in these materials is important for further development of supercapacitors. Here we review recent progress, from both in situ experiments and advanced simulation techniques, in understanding the charge storage mechanism in carbon- and oxide-based supercapacitors. We also discuss the challenges that still need to be addressed for building better supercapacitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Conway, B. E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Springer, 1999).
Miller, J. R. & Simon, P. Electrochemical capacitors for energy management. Science 321, 651–652 (2008).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763 (2006). This paper demonstrates that microporous carbons can be used to maximize the capacitance, owing to the desolvation of the ions in subnanometre pores.
Toupin, M., Brousse, T. & Bélanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 16, 3184–3190 (2004). This paper shows that MnO2-based supercapacitors can achieve very high specific capacitances highlighting the importance of pseudocapacitive mechanisms.
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nature Mater. 7, 845–854 (2008).
Béguin, F., Presser, V., Balducci, A. & Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 26, 2219–2251 (2014).
Raccichini, R., Varzi, A., Passerini, S. & Scrosati, B. The role of graphene for electrochemical energy storage. Nature Mater. 14, 271–279 (2015).
Armand, M., Endres, F., MacFarlane, D. R., Ohno, H. & Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nature Mater. 8, 621–629 (2009).
Brandt, A., Pohlmann, S., Varzi, A., Balducci, A. & Passerini, S. Ionic liquids in supercapacitors. MRS Bull. 38, 554–559 (2013).
Helmholtz, H. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Ann. Phys. Chem. 165, 211–233 (1853).
Eliad, L., Salitra, G., Soffer, A. & Aurbach, D. Ion sieving effects in the electrical double layer of porous carbon electrodes: estimating effective ion size in electrolytic solutions. J. Phys. Chem. B 105, 6880–6887 (2001).
Eliad, L., Salitra, G., Soffer, A. & Aurbach, D. On the mechanism of selective electroadsorption in the pores of carbon molecular sieves. Langmuir 21, 3198–3202 (2005).
Wang, S., Minami, D. & Kaneko, K. Comparative pore structural analysis of highly porous graphene monoliths treated at different temperatures with adsorption of N2 at 77.4 K and of Ar at 87.3 K and 77.4 K. Micropor. Mesopor. Mater. 209, 72–78 (2015).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 87, 1051–1069 (2015).
Kaneko, K., Ishii, C., Ruike, M. & Kuwabara, H. Origin of superhigh microcrystalline graphitic structures of activated carbons. Carbon 30, 1075–1088 (1992).
Setoyama, N., Suzuki, T. & Kaneko, K. Simulation study on the relationship between a high resolution αs-plot and the pore size distribution for activated carbon. Carbon 36, 1459–1467 (1998).
Neimark, A. V., Lin, Y., Ravikovitch, P. I. & Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 47, 1617–1628 (2009). This paper provides the theoretical basis of density functional theory, which is now the most used method for characterizing the surface area of microporous carbons.
Centeno, T. A., Sereda, O. & Stoeckli, F. Capacitance in carbon pores of 0.7 to 15 nm: a regular pattern. Phys. Chem. Chem. Phys. 13, 12403–12406 (2011).
Bandosz, T. J. et al. in Chemistry and Physics of Carbon 41–228 (Marcel Dekker, 2001).
Bousige, C. et al. Realistic molecular model of kerogen's nanostructure. Nature Mater. 15, 576–582 (2016).
Forse, A. C. et al. New insights into the structure of nanoporous carbons from NMR, Raman, and pair distribution function analysis. Chem. Mater. 27, 6848–6857 (2015).
Palmer, J. C. et al. Modeling the structural evolution of carbide-derived carbons using quenched molecular dynamics. Carbon 48, 1116–1123 (2010).
Palmer, J. C. & Gubbins, K. E. Atomistic models for disordered nanoporous carbons using reactive force fields. Micropor. Mesopor. Mater. 154, 24–37 (2012).
Wang, H. et al. Real-time NMR studies of electrochemical double-layer capacitors. J. Am. Chem. Soc. 133, 19270–19273 (2011).
Griffin, J. M. et al. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nature Mater. 14, 812–819 (2015). This paper shows, by combining in situ electrochemical and spectroscopy techniques that different ion adsorption mechanisms can dominate the charging process of supercapacitors depending on the polarization of the electrode.
Boukhalfa, S. et al. In-situ small angle neutron scattering revealing ion sorption in microporous carbon electrical double layer capacitors. ACS Nano 8, 2495–2503 (2014).
Bañuelos, J. L. et al. Densification of ionic liquid molecules within a hierarchical nanoporous carbon structure revealed by small angle scattering and molecular dynamics simulation. Chem. Mater. 26, 1144–1153 (2014).
Deschamps, M. et al. Exploring electrolyte organization in supercapacitor electrodes with solid-state NMR. Nature Mater. 12, 351–358 (2013).
Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nature Mater. 11, 306–310 (2012). This paper provides the first quantitative picture of the structure of an ionic liquid adsorbed inside realistically modelled microporous carbon electrodes.
Merlet, C. et al. Highly confined ions store charge more efficiently in supercapacitors. Nature Commun. 4, 2701 (2013).
Shim, T. & Kim, H. J. Nanoporous carbon supercapacitors in an ionic liquid: a computer simulation study. ACS Nano 4, 2345–2355 (2010).
Merlet, C., Forse, A. C., Griffin, J., Frenkel, D. & Grey, C. P. Lattice simulation method to model diffusion and NMR spectra in porous materials. J. Chem. Phys. 142, 094701 (2015).
Boukhalfa, S., He, L., Melnichenko, Y. B. & Yushin, G. Small-angle neutron scattering for in situ probing of ion adsorption inside micropores. Angew. Chem. Int. Ed. 52, 4618–4622 (2013).
Kondrat, S. & Kornyshev, A. Pressing a spring: what does it take to maximize the energy storage in nanoporous supercapacitors? Nanoscale Horiz. 1, 45–52 (2016).
Largeot, C. et al. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008).
Galhena, D. T., Bayer, B. C., Hofmann, S. & Amaratunga, G. A. Understanding capacitance variation in sub-nanometer pores by in situ tuning of interlayer constrictions. ACS Nano 10, 747–754 (2016).
Raymundo-Pinero, E., Kierzek, K., Machnikowski, J. & Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 44, 2498–2507 (2006).
Levi, M. D., Sigalov, S., Aurbach, D. & Daikhin, L. In situ electrochemical quartz crystal admittance methodology for tracking compositional and mechanical changes in porous carbon electrodes. J. Phys. Chem. C 117, 14876–14889 (2013).
Levi, M. D., Salitra, G., Levy, N., Aurbach, D. & Maier, J. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage. Nature Mater. 8, 872–875 (2009). This paper demonstrates that it is possible to monitor the gravimetric response of microporous carbons during the ion adsorption inside the pores.
Sigalov, S., Levi, M. D., Daikhin, L., Salitra, G. & Aurbach, D. Electrochemical quartz crystal admittance studies of ion adsorption on nanoporous composite carbon electrodes in aprotic solutions. J. Solid State Electrochem. 18, 1335–1344 (2014).
Ohkubo, T. et al. Restricted hydration structures of Rb and Br ions confined in slit-shaped carbon nanospace. J. Am. Chem. Soc. 124, 11860–11861 (2002). This paper shows that desolvation of aqueous ions occurs under extreme confinement using extended X-ray absorption fine structure experiments.
Tsai, W.-Y., Taberna, P.-L. & Simon, P. Electrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons. J. Am. Chem. Soc. 136, 8722–8728 (2014).
Fedorov, M. V. & Kornyshev, A. A. Ionic liquids at electrified interfaces. Chem. Rev. 114, 2978–3036 (2014).
Xing, L., Vatamanu, J., Borodin, O. & Bedrov, D. On the atomistic nature of capacitance enhancement generated by ionic liquid electrolyte confined in subnanometer pores. J. Phys. Chem. Lett. 4, 132–140 (2013).
Freise, V. Zur theorie der diffusendoppeltschicht. Z. Elektrochem. 56, 822–827 (1952).
Kornyshev, A. A. Double-layer in ionic liquids: paradigm change? J. Phys. Chem. B 111, 5545–5557 (2007).
Limmer, D. T. et al. Charge fluctuations in nanoscale capacitors. Phys. Rev. Lett. 111, 106102 (2013).
Merlet, C. et al. The electric double layer has a life of its own. J. Phys. Chem. C 118 18291–18298 (2014).
Kornyshev, A. A. & Qiao, R. Three-dimensional double layers. J. Phys. Chem. C 118, 18285–18290 (2014).
Gebbie, M. A. et al. Ionic liquids behave as dilute electrolyte solutions. Proc. Natl Acad. Sci. USA 110, 9674–9679 (2013).
Perkin, S., Salanne, M., Madden, P. & Lynden-Bell, R. Is a stern and diffuse layer model appropriate to ionic liquids at surfaces? Proc. Natl Acad. Sci. USA 110, E4121 (2013).
Bozym, D. et al. Anomalous capacitance maximum of the glassy carbon–ionic liquid interface through dilution with organic solvents. J. Phys. Chem. Lett. 6, 2644–2648 (2015).
Lee, A. A., Vella, D., Perkin, S. & Goriely, A. Are room-temperature ionic liquids dilute electrolyte? J. Phys. Chem. Lett. 6, 159–163 (2015).
Bazant, M. Z., Storey, B. D. & Kornyshev, A. A. Double layer in ionic liquids: overscreening versus crowding. Phys. Rev. Lett. 106, 046102 (2011).
Kondrat, S. & Kornyshev, A. A. Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys. Condens. Matter 23, 022201 (2011). This paper explains the increase of capacitance in microporous carbons by the formation of image charges on the walls which screen the electrostatic interactions between the ions, leading to the formation of a ‘superionic’ state.
Kondrat, S., Georgi, N., Fedorov, M. V. & Kornyshev, A. A. A superionic state in nano-porous double-layer capacitors: insights from Monte Carlo simulations. Phys. Chem. Chem. Phys. 13, 11359–11366 (2011).
Griffin, J. M. et al. Ion counting in supercapacitor electrode using NMR spectroscopy. Faraday Discuss. 176, 49–68 (2014).
Pean, C. et al. Confinement, desolvation and electrosorption effects on the diffusion of ions in nanoporous carbon electrodes. J. Am. Chem. Soc. 137, 12627–12632 (2015).
Richey, F. W., Dyatkin, B., Gogotsi, Y. & Elabd, Y. A. Ion dynamics in porous carbon electrodes in supercapacitors using in situ infrared spectroelectrochemistry. J. Am. Chem. Soc. 135, 12818–12826 (2013).
Prehal, C. et al. Tracking the structural arrangement of ions in carbon supercapacitor nanopores using in-situ small angle X-ray scattering. Energy Environ. Sci. 8, 1725–1735 (2015).
Ilott, A. J., Trease, N. M., Grey, C. P. & Jerschow, A. Multinuclear in situ magnetic resonance imaging of electrochemical double-layer capacitors. Nature Commun. 5, 4536 (2014).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nature Mater. 13, 387–393 (2014).
He, Y. et al. The importance of ion packing on the dynamics of ionic liquids during micropore charging. J. Phys. Chem. Lett. 7, 36–42 (2016).
Pean, C. et al. On the dynamics of charging in nanoporous carbon-based supercapacitors. ACS Nano 8, 1576–1583 (2014).
Augustyn, C., Simon, P. & Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014).
Kim, J. W., Augustyn, V. & Dunn, B. The effect of crystallinity on the rapid pseudocapacitive response of Nb2O5 . Adv. Energy Mater. 2, 141–148 (2012).
Come, J. et al. Electrochemical kinetics of nanostructured Nb2O5 electrodes. J. Electrochem. Soc. 161, A718–A725 (2014).
Dmowski, W., Egami, T., Swider-Lyons, K. E., Love, C. T. & Rolison, D. R. Local atomic structure and conduction mechanism of nanocrystalline hydrous RuO2 from X-ray scattering. J. Phys. Chem. B 106, 12677–12683 (2002).
Augustyn, V. et al. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nature Mater. 12, 518–522 (2013). This paper reports on a pseudocapacitance mechanism based on lithium ion intercalation and defines the structural characteristics which are necessary for this process to occur.
Brousse, T., Belanger, D. & Long, J. W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 162, A5185–A5189 (2015).
Kim, H.-S., Cook, J. B., Tolbert, S. H. & Dunn, B. The development of pseudocapacitive properties in nanosized-MoO2 . J. Electrochem. Soc. 162, A5083–A5090 (2015).
Simon, P., Gogotsi, Y. & Dunn, B. Where do batteries end and supercapacitors begin? Science 343, 1210–1211 (2014).
Augustyn, V. et al. Lithium-ion storage properties of titanium oxide nanosheets. Mater. Horiz. 1, 219–233 (2014).
Athouel, L. et al. Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte. J. Phys. Chem. C 112, 7270–7277 (2008).
Wei, W., Cui, X., Chen, W. & Ivey, D. G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 40, 1697–1721 (2011).
Ghodbane, O., Pascal, J.-L. & Favier, F. Microstructural effects on charge-storage properties in MnO2-based electrochemical supercapacitors. ACS Appl. Mater. Interfaces 1, 1130–1139 (2009).
Ghodbane, O., Ataherian, F., Wu, N.-L. & Favier, F. In situ crystallographic investigations of charge storage mechanisms in MnO2-based electrochemical capacitors. J. Power Sources 206, 454–462 (2012).
Rangom, Y., Tang, X. & Nazar, L. F. Carbon nanotube-based supercapacitors with excellent ac line filtering and rate capability via improved interfacial impedance. ACS Nano 9, 7248–7255 (2015).
Zhu, Y. et al. A carbon quantum dot decorated RuO2 network: outstanding supercapacitances under ultrafast charge and discharge. Energy Environ. Sci. 6, 3665–3675 (2013).
Aravindan, V., Gnanaraj, J., Lee, Y. S. & Madhavi, S. Insertion-type electrodes for nonaqueous Li-ion capacitors. Chem. Rev. 114, 11619–11635 (2014).
Naoi, K., Ishimoto, S., Isobe, Y. & Aoyagi, S. High-rate nano-crystalline Li4Ti5O12 attached on carbon nano-fibers for hybrid supercapacitors. J. Power Sources 195, 6250–6254 (2010).
Naoi, K., Ishimoto, S., Ogihara, N., Nakagawa, Y. & Hatta, S. Encapsulation of nanodot ruthenium oxide into KB for electrochemical capacitors. J. Electrochem. Soc. 156, A52–A59 (2009).
Naoi, K., Ishimoto, S., Miyamoto, J. & Naoi, W. Second generation ‘nanohybrid supercapacitor’: evolution of capacitive energy storage devices. Energy Environ. Sci. 5, 9363–9373 (2012). This paper reports on the perspectives opened by combining a negative graphite electrode of a Li-ion battery with a capacitive porous-carbon positive electrode.
Naoi, K., Naoi, W., Aoyagi, S., Miyamoto, J. & Kamino, T. New generation ‘nanohybrid supercapacitor’. Acc. Chem. Res. 46, 1075–1083 (2012).
Vatamanu, J. & Bedrov, D. Capacitive energy storage: current and future challenges. J. Phys. Chem. Lett. 6, 3594–3609 (2015).
Lin, T. et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 350, 1508–1513 (2015).
Curtarolo, S. et al. The high-throughput highway to computational materials design. Nature Mater. 12, 191–201 (2013).
Schutter, C., Husch, T., Korth, M. & Balducci, A. Toward new solvents for EDLCs: from computational screening to electrochemical validation. J. Phys. Chem. C 119, 13413–13424 (2015).
Pognon, G., Brousse, T., Demarconnay, L. & Bélanger, D. Performance and stability of electrochemical capacitor based on anthraquinone modified activated carbon. J. Power Sources 196, 4117–4122 (2011).
Abbas, Q. et al. Strategies to improve the performance of carbon/carbon capacitors in salt aqueous electrolytes. J. Electrochem. Soc. 162, A5148–A5157 (2015).
Pohlmann, S. et al. Mixtures of azepanium based ionic liquids and propylene carbonate as high voltage electrolytes for supercapacitors. Electrochim. Acta 153, 426–432 (2015).
Acerce, M., Voiry, D. & Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nature Nanotech. 10, 313–318 (2015).
Ghidiu, M., Lukatskaya, M. R., Zhao, M. Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014).
Shi, C. Y. et al. Structure of nanocrystalline Ti3C2 MXene using atomic pair distribution function. Phys. Rev. Lett. 112, 125501 (2014).
Levi, M. D. et al. Solving the capacitive paradox of 2D MXene using electrochemical quartz-crystal admittance and. in situ electronic conductance measurements. Adv. Energ. Mater. 5, 1400815 (2015).
Soloveichik, G. L. Flow batteries: current status and trends. Chem. Rev. 115, 11533–11558 (2015).
Kwon, C.-H. et al. High power biofuel cells textiles from woven biscrolled carbon nanotube yarns. Nature Commun. 5, 3928 (2014).
Presser, V. et al. The electrochemical flow capacitor: a new concept for rapid energy storage and recovery. Adv. Energ. Mater. 2, 895–902 (2012).
Brogioli, D. Extracting renewable energy from a salinity difference using a capacitor. Phys. Rev. Lett. 103, 058501 (2009).
Siria, A. et al. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 494, 455–458 (2013).
Wang, J. Carbon nanotube based electrochemical biosensors. Electroanalysis 17, 7–14 (2005).
Mirica, K. A., Azzarelli, J. M., Weis, J. G., Schnorr, J. M. & Swager, T. M. Rapid prototyping of carbon-based chemiresistive gas sensors on paper. Proc. Natl Acad. Sci. USA 110, E3265–E3270 (2013).
Acknowledgements
We thank our many co-workers who have contributed to the work presented in this review. C.P.G. thanks J. Griffin for his critical reading of the manuscript. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ ERC grant agreement no. 102539.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Salanne, M., Rotenberg, B., Naoi, K. et al. Efficient storage mechanisms for building better supercapacitors. Nat Energy 1, 16070 (2016). https://doi.org/10.1038/nenergy.2016.70
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.70
This article is cited by
-
Porous carbon from polyvinylidene chloride or polyvinylidene fluoride with ZnO, Mg(OH)2, and KOH for supercapacitor
Carbon Letters (2024)
-
Ionic liquids in green energy storage devices: lithium-ion batteries, supercapacitors, and solar cells
Monatshefte für Chemie - Chemical Monthly (2024)
-
Free-standing β-Ta2O5/SWCNTs composite film for high-rate Li-ion storage
Science China Technological Sciences (2024)
-
A pre-burning treatment facilitating formation of pores in biochars and reinforcing their electrochemical performance in supercapacitor
Ionics (2024)
-
In situ monitoring redox processes in energy storage using UV–Vis spectroscopy
Nature Energy (2023)